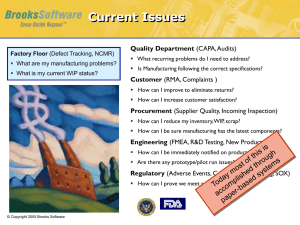
Six Methods for Verifying the effectiveness of corrective and
advertisement

Six Methods for Verifying the effectiveness of corrective and preventive actions. How to choose the right method and time frame Verifying the effectiveness of corrective and preventive actions (CAPAs) closes the loop between identifying a problem and completing the actions to solve it. It’s reasonable to assume that if a problem is worth solving, it’s also worth verifying that the solution worked. However, given the wide range of problems that could occur, determining the best verification approach and time frame to implement it can often seem elusive. Before we discuss CAPA effectiveness, we need to look at a few of the reasons why performing this check is often a challenge. Here are six: 1. The problem isn’t well defined 2. The root cause isn’t determined 3. It’s not really a CAPA Sometimes breaking the mental logjam is as simple as asking, “What problem were we trying to solve?” That sounds like an easy question, but when the answer isn’t well defined or stated simply, measuring success isn’t easy to do. This is a natural consequence of the first reason. It’s next to impossible to determine the root cause for a fuzzy problem, or one that seems too complicated to explain. Those who try also get a fuzzy root cause. All too often, the CAPA system has become a quality work-order system (aka dumping ground) because the commonly used data management systems, provide project management structure and visibility. But without a stated problem or determining a root cause, it’s not a CAPA; it’s just a project. 1 4. CAPA effectiveness verification is used for everything 5. We over-think it 6. It’s not considered important CAPA effectiveness verification can be too much of a good thing when it’s expected for every possible CAPA. This usually occurs from the cascading problem of a CAPA being required for every deviation, and a deviation being required for every conceivable blip. Soon you become a drowning victim of your own making. Rather than allowing reason to prevail, there are those who tend to complicate just about everything. Determining and applying an appropriate verification method is no exception. Yes, we operate in a scientific environment, but not every method of verifying effectiveness must be labor-intensive. Major processes need not be applied to minor problems. There are those who believe that living with an ongoing problem is the path of least resistance, especially when compared to conducting the same boilerplate investigation (i.e., same problem, different day) in order to get on with the real work of production. Having a low tolerance for recurring problems is truly the root cause for many who are treading water in a deviation-swirling tide pool. Assuming we have a real CAPA, where an investigation was conducted on a well-defined problem to determine the root cause and product impact, we can turn to the regulatory requirements and business obligation to evaluate how well we spent our resources to eliminate the problem permanently. This brings us to options for verifying CAPA effectiveness. Here are six:performing this check is often a challenge. Here are six: 1. 2. Auditing is used when the solution involves changes to a system and verification is done to see whether the changes are in place procedurally and in use behaviorally. An example is an audit of a new-line clearance checklist to verify effective implementation of the checklist. Spot check is used for random observations of performance or reviews of records. Spot checks provide immediate but limited feedback. An example is a spot-check of batch records to ensure that the pH step was performed correctly after training on the new procedure. 2 3. 4. 5. Sampling 6. Periodic product review is the retrospective review of data to is used for observations of variables or attributes, per defined sampling plan. An example of sampling a statistical sample randomly drawn from lot XYZ123 to confirm that a defect has been removed after implementing a process improvement. Monitoring iis used for real-time observations over a defined period. An example of monitoring is real-time observation to verify that changes to operator-owning practices were implemented. Trend analysis is the retrospective review of data to verify that expected results were achieved. An example of trend analysis is the review of environmental monitoring (EM) data for the period covering the last 30 batches to show the downward trend in EM excursions due to process improvements. verify that expected results were achieved. An example of trend analysis is the review of environmental monitoring (EM) data for the period covering the last 30 batches to show the downward trend in EM excursions due to process improvements. Now that we have a real CAPA and a selected a method to verify its effectiveness, we must determine an appropriate time frame to perform the verification. Time frames are subjective, but there must be a basis for the decision. Here are points to consider when determining an appropriate time frame for CAPA effectiveness verification. Allow relatively less time after implementing the solution when there is: • Higher opportunity for occurrence and observation • Higher probability of detection • An engineered solution In these cases, fewer observations are needed for a high degree of confidence. • • • • Allow relatively more time after implementing the solution when there is: Lower opportunity for occurrence and observation Lower probability of detection A behavioral or training solution In these cases, more observations are needed for a high degree of confidence. http://theqapharm.blogspot.com.au/2015/03/6-methods-for-capa-effectiveness.html 3