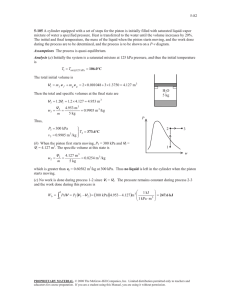
2.46 A piston/cylinder with a cross-sectional area of 0.01 m3 has a
advertisement

2.46 A piston/cylinder with a cross-sectional area of 0.01 m3 has a mass of 100 kg resting on the stops as shown in the figure. With an outside atmospheric pressure of 100 kPa what should the water pressure be to lift the piston? Given: P0 = 100 kPa, mpiston = 100 kg, Apiston = 0.01 m2 . Find: The water pressure needed to lift the piston. Assumptions: There is negligible friction between the piston and the cylinder. The area of the stops is negligible. All parameters are know to three significant digits. Solution: Sum the forces on the piston. Σf = 0 = Pwater Apiston − P0 Apiston − mpiston g mpiston g Pwater = P0 + Apiston N (100 kg) 9.81 kg Pwater = 100 kPa + 0.01 m2 Pwater = 100 kPa + 98100 Pa Pwater = 198 kPa 1 2.47 a 5-kg cannonball acts as a piston in a cylinder with a diameter of 0.15 m. As the gunpowder is burned, a pressure of 7 MPa is created in the gas behind the ball. What is the acceleration of the ball if the cylinder (cannon) is pointing horizontally? Given: Pressure inside of cannon, P = 7 MPa. Diameter of cannon, D = 0.15 m. Mass of cannonball, mball = 5 kg. Find: The acceleration of the cannonball if the cannon is horizontal. Assumptions: Negligible friction between cannonball and cannon. All parameters are know to three significant digits. Solution: Sum the forces. Σf = ma Pcannon Acannon − Patmosphere Acannon = mcannonball acannonball acannonball = acannonball acannonball acannonball acannonball 2 (Pcannon − Patmosphere ) Acannon mcannonball (Pcannon − Patmosphere ) πD 4 = mcannonball 2 m) (7.00 MPa − 101 kPa) π(0.15 4 = 5.00 kg (6.9 MPa) 0.0177 m2 = 5.00 kg m = 2.44 × 104 s 2 2.71 A U-tube manometer filled with water (density=1000 kg/m3 ) shows a height difference of 25 cm. what is the gauge pressure? If the right branch is tilted to make and angle of 30◦ with the horizontal, as shown in the the figure, what should the length of the column in the tilted tube be relative to the U-tube? Given: Height difference of a manometer, h = 25 cm. Find: The gauge pressure. The length of titled water column. Assumptions: Solution: ∆P = ρg∆z kg N ∆P = 1000 3 9.81 0.25 m m kg ∆P = 2450 Pa = 2.45 kPa Use trigonometry to find L. sin (θ) = h h 0.25 m ⇒L= (θ) = = 0.500 m L sin 0.5 3 2.88 Two cylinders are connected by a piston as shown in the figure. Cylinder A is used as a hydraulic lift and pumped to 500 kPa. The piston has a mass of 25 kg, and there is standard gravity. What is the gas pressure in cylinder B? Given: Pressure in A, PA = 500 kPa. Atmospheric pressure, P0 = 100 kPa. Diameter at A, DA = 100 mm. Diameter at B, DB = 25 mm. Mass of piston, mp = 25 kg. Find: Pressure in B Assumptions: Negligible friction between piston and cylinders. Solution: Sum the forces Σf = 0 = PA AA − PB AB − P0 (AA − AB ) − mp g PB = [PA AA − P0 (AA − AB ) − mp g] /AB 2 AA = πDA /4 = π (0.100 m)2 /4 = 7.85 × 10−3 m2 2 2 AB = πDB /4 = π (0.025 m) /4 = 4.91 × 10−5 m2 AA − AB = 7.36 × 10−3 m2 N /4.91 × 10−5 m2 PB = (500 kPa) 7.85 × 10−3 m2 − (100 kPa) 7.36 × 10−3 m2 − (25 kg) 9.81 kg PB = 6.00 MPa 4 3.27 Determine whether water at each of the following states is a compressed liquid, a superheated vapor, or a mixture of saturated liquid and vapor: a. 10 MPa, 0.003 m3 /kg b. 1 MPa, 190◦ C c. 200◦ C, 0.1 m3 /kg d. 10 kPa, 10◦ C Solution: a. 10 MPa, 0.003 m3 /kg 3 m3 From table B.1.2 with P=10000 kPa vf = 0.001452 m kg , vg = 0.01803 kg . The given specific volume is between vf and vg so the state is a saturated mixture. b. 1 MPa, 190◦ C From table B.1.2 with P=1000 kPa Tt extsat = 179.91◦C. The given temperature is above the saturation temperature so the state is superheated. 3 3 m c. 200◦ C, 0.1 m3 /kg From table B.1.1 with P=200◦C vf = 0.001156 m kg , vg = 0.12736 kg . The given specific volume is between vf and vg so the state is a saturated mixture. d. 10 kPa, 10◦ CFrom table B.1.2 with P=10 kPa Tt extsat = 45.81◦ C. The given temperature is below the saturation temperature so the state is a compressed (or sub-cooled) liquid. 5 3.28 For water at 100 Kpa with a quality of 10%, find the volume fraction of vapor. Given: Pressure=100 kPa, x = 0.1 Find: Volume fraction of vapor, VVv Solution: There are lots of ways to solve this...here is one way... First some algebra, V = mv Vv = mv vg Vl = ml vf v = xvg + (1 − x)vf mv vg mv vg Vv = = V mv m v Now look up need values on table B.1.2 and perform calculations. vf = 0.001043 vg = 1.694 m3 kg m3 kg m3 m3 m3 + (0.9) 0.001043 = 0.170 kg kg kg ! m3 1.694 kg mv vg Vv = 0.994 ⇒ 99.4% = = (0.1) 3 Vl m v 0.170 m kg v = (0.1) 1.694 6 3.43 Water at 120◦ C with a quality of 25% has its temperature raised by 20◦ C in a constant-volume process. What is the new quality and pressure. Given: Initial state of water (T1 = 120◦ C, x = 0.25). Final temperature T2 = 140◦ C. Constant volume V2 = V1 Find: Final quality and pressure Assumptions: No mass enters or leaves the system. Solution: Define states: State 1 State 2 T1 = 120◦ C T2 = 140◦ C x1 = 0.25 v2 = v1 Calculate the initial volume. v1 = (x) vg + (1 − x) vf ⇐ Look up vf and vg on table B.1.1 with T=120◦ C. = (0.25) 0.89186 = 0.2238 m3 m3 + (0.75) 0.001060 kg kg m3 ⇐ This is also v2 kg 3 3 m From table B.1.1 with T=140◦C vf = 0.001080 m kg and vg = 0.50885 kg . v2 is between vf and vg so state 2 is a saturated mixture. P2 = Psat |T =140◦ C = 361kPa 3 3 m 0.2238 m v2 − vf kg − 0.001080 kg = 0.438 x2 = = 3 m3 vg − vf 0.50885 m kg − 0.001080 kg 7 3.47 Water at 200 kPa with a quality of %25 has its temperature raised 20◦ Cin a constant-pressure process. What is the new quality and volume? Given: Initial state given, P1 = 200 kPa, x = 0.25. Pressure does not change, P2 = 200 kPa. Temperature increases, T2 = T1 + 20◦ C. Assumptions: No mass enter or leaves the system. Define states: State 1 State 2 P1 = 200 kPa P2 = 200 kPa x1 = 0.25 T2 = T1 + 20◦ C Determine the final temperature. T1 = Tsat |P =200 kPa = 120.2◦C T2 = 140.2◦C T2 is greater than the saturation temperature at 200 kPa so the final state is superheated. Quality is not defined for superheated vapor (x =N/A). Use table B.1.3 with P=200 kPa to find the final specific volume. 3 P=200 kPa T(◦ C) v( m kg ) A 120.23 0.88573 B 150 0.95964 v − vA T − TA = vB − vA TB − TA T − TA v= (vB − vA ) + vA TB − TA 140.2◦C − 120.23◦C m3 m3 m3 v= 0.95964 + 0.88573 − 0.88573 ◦ ◦ 150 C − 120.23 C kg kg kg 3 m v = 0.935 kg 8