TER-POGOSSIAN JOHN O. EICHLING, MARCUS E. RAICHLE
advertisement

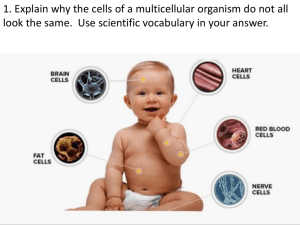
Evidence of the Limitations of Water as a Freely Diffusible Tracer in Brain of the Rhesus
Monkey
JOHN O. EICHLING, MARCUS E. RAICHLE, ROBERT L. GRUBB, Jr. and MICHEL M.
TER-POGOSSIAN
Circ Res. 1974;35:358-364
doi: 10.1161/01.RES.35.3.358
Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231
Copyright © 1974 American Heart Association, Inc. All rights reserved.
Print ISSN: 0009-7330. Online ISSN: 1524-4571
The online version of this article, along with updated information and services, is located on the
World Wide Web at:
http://circres.ahajournals.org/content/35/3/358
Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in
Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the
Editorial Office. Once the online version of the published article for which permission is being requested is
located, click Request Permissions in the middle column of the Web page under Services. Further information
about this process is available in the Permissions and Rights Question and Answer document.
Reprints: Information about reprints can be found online at:
http://www.lww.com/reprints
Subscriptions: Information about subscribing to Circulation Research is online at:
http://circres.ahajournals.org//subscriptions/
Downloaded from http://circres.ahajournals.org/ by guest on February 23, 2013
Evidence of the Limitations of Water as a Freely Diffusible
Tracer in Brain of the Rhesus Monkey
By John O. Eichling, Marcus E. Raichle, Robert L. Grubb, Jr., and Michel M. Ter-Pogossian
ABSTRACT
The extraction of 16O-labeled water by the brain during a single capillary transit was
studied in vivo in 20 adult rhesus monkeys by external detection of the time course of the
tracer subsequent to the internal carotid injection of 0.2 ml of whole blood labeled with
H,1BO. The data showed that labeled water does not freely equilibrate with the
exchangeable water in the brain when the mean cerebral blood flow exceeds 30 ml/100 g
min"1. At the normal cerebral blood flow in the rhesus monkey (~50 ml/100 g min~'), only
90% of the H215O is extracted during a single capillary transit. In addition, cerebral blood
flow was determined with H,1SO and "*Xe in these monkeys using residue detection and
employing the central volume principle. The data supported the hypothesis that a
diffusible tracer, H,15O, need not be in complete equilibrium between the phases of a
system for the application of the central volume principle to be valid. Finally, the brain
capillary permeability-surface area product was computed from these data; it was
approximately 0.023 cm'/sec g-1.
KEY WORDS
blood-brain barrier
cerebral blood
flow
'"Xe
central volume principle
capillary permeability
"O-labeled water
• The diffusion of radioisotopically labeled water
into tissue is assumed to be rapid enough relative
to bulk movement (convection) by capillary blood
flow through the tissue to allow complete equilibration during a single capillary transit and subsequent
clearance from the tissue. In effect, the movement
of water or a similar tracer through a given tissue is
assumed to be blood flow limited (1). Evidence for
this assumption comes from the work of Johnson et
al. (2), who have shown that water is flow limited in
its exchange in dog heart and skeletal muscle. In
addition, Yipintsoi and Bassingthwaighte (3) have
shown that labeled water is flow limited in its
capillary exchange in dog heart. Yudilevich and
DeRose (4) have concluded that labeled water
diffuses freely in dog brain. Others have also
assumed that water attains complete equilibrium
with the exchangeable water in the brain during a
single capillary transit (5-9). As a result, labeled
water has been used as a standard for the evaluation of blood-brain barrier transport and brain distribution of other substances and as a tracer for
From the Division of Radiation Sciences, The Edward
Mallinckrodt Institute of Radiology, and the Department of
Neurology, Division of Neurosurgery, Washington University
School of Medicine, St. Louis, Missouri 63110.
This work was supported by U. S. Public Health Service
Grants 5 POl HL13851, RR 00396, and 5 PO1 NSO 6833 from
the National Institutes of Health and by Teacher-Investigator
Award 1 F l l NS11059 (Dr. Raichle) from the National Institute
of Neurological Disease and Stroke.
Received February 13, 1974. Accepted for publication May
15, 1974.
358
the measurement of cerebral blood flow (10). In
the present paper, we will present evidence that
labeled water does not freely equilibrate with the
exchangeable water in the brain of the rhesus monkey when the mean cerebral blood flow exceeds
approximately 30 ml/100 g min" 1 .
Methods
The fraction of labeled water extracted by the brain
during a single capillary transit was determined subsequent to the internal carotid injection of 0.2 ml of whole
blood labeled with H,'*O in 20 adult rhesus monkeys. To
facilitate the injection of the radioisotope into the
internal carotid artery, all branches of the right external
carotid artery were ligated 2 weeks prior to the experiments. The radioisotope was then injected into the
common carotid artery through a small (diameter 0.021
cm) catheter positioned there under fluoroscopic control
from the femoral artery.
The monkeys were anesthetized with phencyclidine,
paralyzed with gallamine, and passively ventilated with
100% O,. End-tidal Pco,, arterial blood pressure, and
rectal temperature were continuously monitored. Temperature was maintained between 37°C and 39°C with a
heating pad. Arterial pH, Pco,, and Po, were measured before and after each injection.
The time course of tracer movement through the
injected hemisphere was monitored by a 3 x 2-inch
Nal(Tl) scintillation detector appropriately collimated
and positioned to ensure essentially uniform detection
efficiency for the entire hemisphere. The signal from the
detector was processed by a pulse-height analyzer with
an energy window of acceptance adjusted symmetrically
around the 511-kev photopeak of H,"O (481-541 kev) to
eliminate scattered radiation. The accepted events
(counts) per time frame were stored in the memory of a
Circulation Research, Vol. 35, September 1974
Downloaded from http://circres.ahajournals.org/ by guest on February 23, 2013
359
DIFFUSION LIMITATIONS OF WATER
small laboratory computer (LINC). Appropriate data
processing including conversion to count rate (counts/
sec) as a function of time and corrections of the count
rate for electronic dead time loss, physical decay, and
background were performed by the computer. Routine
data retrieval was in the form of processed count rate as a
function of time plotted by an x-y plotter. Optimal
temporal resolution was achieved in the initial portion of
each recording by utilizing sampling integration times of
0.1 seconds. Statistically smooth recordings were ensured
by injecting enough labeled water (~200 uc/0.2 ml) to
achieve a peak counting rate of 10,000-20,000 counts/sec.
Radioactive water labeled with ">O (half-life 123
seconds) was produced for these studies in the Washington University Medical School Cyclotron by deuteron
bombardment of nitrogen gas (11).
The fraction of labeled water extracted by the brain
during a single capillary transit (E) was determined by
graphically extrapolating the relatively slow clearance of
labeled tissue water back to the abscissa of the maximum of the perfusion peak and computing the ratio
E = B/A,
(1)
as shown in Figure 1.
Cerebral blood flow was determined by utilizing residue detection (12) of the bolus of labeled water injected
SOK r
IOK
Q
Z
o
u
-O.SI tec (H f °O)
IK
T1/l~O.53»ec (C°O-hemoglobin)
IOO
5
10
TIME
19
dec)
FIGURE 1
Semilogarithmic recordings obtained subsequent to serial intracarotid injections of H,"O (top) and C"O-labeled hemoglobin (bottom). Extrapolation of the relatiuely slow clearance of
labeled tissue water back to the abscissa of the maximum of the
perfusion peak allows the computation of the extraction fraction: E - B/A. Resolution of the water clearance into two
components shows that the half-time (TJ4) of the fast component is essentially that of labeled red cells.
into the internal carotid artery. The time-activity curve
for the washout of H,"O from the brain was used to
calculate the water mean transit time (TH^,), which is
defined as
Jo" q(t)dt
7=
(2)
where q(t) is the radioactivity level in the region under
study as a function of time and q0 is the dose of
radioactivity in the injected bolus. The computed value
of 7Hin was combined with the central volume principle
(12),
T = V/F,
(3)
where F is the volumetric flow rate of vascular fluid and
V is the "volume of distribution" of the tracer, and the
mean equilibrium brain-blood partition coefficient of
water (\HlO) to yield cerebral blood flow (CBF) in ml/100
g min"1:
CBF =
, x 100
(4)
A value of 0.95 ml/g was used for XHK1.
The extraction fraction (E) of labeled water and the
cerebral blood flow were measured at different levels of
arterial CO, tension. Arterial Pco, was lowered by
passive hyperventilation and raised by ventilation with a
gas mixture of 90% O,-10% CO, (arterial Pco, range 14 to
98 torr). A period of at least 15 minutes was allowed for
the establishment of a steady state at each level of
arterial Pco,.
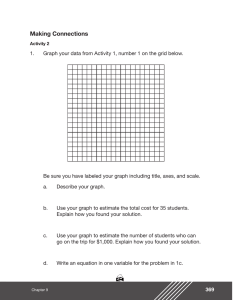
Results
An example of the data obtained is shown in
Figure 1. The early portion of the data (0-5 seconds) reveals the rapid clearance of a fraction of
the injected water from the field of view of the detector. The half-time for the clearance of this rapidly egressing fraction during the first several
seconds following injection was compared with
that of the vascular indicator CO-labeled hemoglobin. C'°O-labeled hemoglobin and water were
injected in sequence; arterial blood gas measurements bridged the injections to ensure a steady
state. The half-times were obtained by conventional curve stripping. The computed half-times
for the rapid component of the water clearance
ranged from 0.5 to 2.5 seconds; in all instances, they
were similar to those of the corresponding vascular
tracer. An example of such a comparison is shown
in Figure 1.
The relationship between E and cerebral blood
flow is shown in Figure 2. These data demonstrate
a highly significant relationship between cerebral
blood flow and E (P« 0.001). The equation for the
linear regression line is E = 1.11 - 0.0037CBF
with a correlation coefficient of r = -0.97. From
these data it can be seen that complete extraction
Circulation Research, Vol. 35, September 1974
Downloaded from http://circres.ahajournals.org/ by guest on February 23, 2013
360
EICHLING, RAICHLE. GRUBB. TER POGOSSIAN
t
100
o
4
CE
u. 80
x
60
UJ
0
20
40
CBF
60
80
100
120
140 —1>
(ml/IOOg/min)
FIGURE 2
Fraction of H,"0 extracted during a single cerebral passage, E,
plotted as a function of the cerebral blood flow (CBF). The
equation of the linear regression line is E - 1.11 - 0.0037CBF (r
= -0.97, P« 0.001).
of water is achieved only for cerebral blood flows
less than approximately 30 ml/100 g min" 1 ; this
value is considerably less than the normal cerebral
blood flow for the rhesus monkey (~50 ml/100 g
min"1) (13).
Discussion
The rapid egress of an appreciable fraction of the
injected labeled water from the field of view of the
detector could be interpreted in several ways. It
could represent (1) an artifact of the experimental
procedure, (2) anatomical arteriovenous shunting
in the brain, (3) a permeability restriction imposed
on the labeled erythrocyte water, or (4) a braincapillary permeability limitation of the water exchange. We have established to our satisfaction
that an appreciable brain-capillary permeability
limitation to water is the only plausible explanation. Negation of the other interpretations was
achieved in the following manner.
Artifact of the Experimental Procedure.—A
rapid redistribution of the labeled water within the
brain could perhaps produce the observed phenomenon if the detection efficiency of our detector
varied appreciably within the monitored field or if
the redistribution of the tracer included tissues not
in the view of the collimated detector. Both possibilities were eliminated by moving the detector 100
cm from the brain, thus radically changing the
detection geometry; no change in E was observed
following this maneuver.
Neither the monkey preparation nor the injection procedure is responsible for our observations.
The observed E was unchanged whether the labeled water was injected into the common carotid
artery of the model hemisphere with the external
carotid artery ligated or into the common carotid
artery of the contralateral hemisphere. Perturbation of cerebral blood flow due to our standard
injection procedure (0.2 ml, 0.5 seconds) seems
unlikely, since 0.05 ml of labeled water injected in
approximately 2 seconds also yielded similar extraction fractions.
Arteriovenous Shunts.—The presence of arteriovenous shunts within the brain could account for
the nonextracted fraction of the injected water.
Evidence suggesting their presence has been reported (14-17). To exclude the presence of arteriovenous shunts within the brain as a possible
explanation for our finding, monkeys were injected
with labeled water and labeled microspheres (8sSr,
514 kev, 15 ± 5^ in diameter) (3M Company) under
identical flow conditions. In addition to monitoring
the brain activity with an external detector, a small
catheter (diameter 0.021 cm) was operatively
placed in the superior sagittal sinus. A second
detector was placed over the external extension of
this indwelling catheter, and blood was drawn
through it at a rate of 2.5 ml/min to record any
radioactivity leaving the brain following the injection of the microspheres. These experiments revealed total extraction of the microspheres by the
brain when the value of E for water was significantly reduced. An example of this observation is
shown in Figure 3.
Erythrocyte Permeability Limitation.—The injected labeled water contained in the whole blood
could be considered to be partitioned into plasma
water and erythrocyte water. The diffusion of the
erythrocyte water into brain tissue could be restricted by the permeability of the erythocyte
membrane. Two observations in our study fail to
support this hypothesis. First, the systemic hematocrit was reduced in one monkey from 0.41 to 0.20
by the acute removal of blood and its replacement
with saline. The observed values of E in this
monkey were those predicted by the values of
cerebral blood flow, as shown in Figure 2. Second,
several injections in additional monkeys of labeled
plasma water, labeled erythrocyte water (packed
cells), and saline containing labeled water resulted
in identical values of E at a given value of cerebral
blood flow. These observations coupled with the
known very high water diffusion permeability of
the erythrocyte (18) make it unlikely that reCirculation Research, Vol. 35, September 1974
Downloaded from http://circres.ahajournals.org/ by guest on February 23, 2013
DIFFUSION LIMITATIONS OF WATER
361
50K
. ' V / ' S 0 - water (E • 65%)
IOK
is
Sr-microiphere»
( E * I O O % )
o
z
o
IK
3
o
o
100
25
15
TIME
35
(sec)
FIGURE 3
Semilogarithmic recordings obtained subsequent to serial intracarotid injections of H,"0 (top) and "Sr-labeled microspheres (bottom). At this elevated cerebral blood flow (~I20
ml/100 g min~l) total extraction of the microspheres was
obtained, although the extraction fraction (E) of the water was
significantly reduced.
stricted diffusion of water across the erythrocyte
membrane is responsible for our observations.
Thus, it appears reasonable to assume that the
capillary wall is the limiting structure to the
diffusion of the labeled water into the brain. The
injected bolus, in effect, divides into two fractions,
a nonextracted or transmitted fraction which is
confined to the vascular volume and an extracted
fraction which passes through the capillary wall,
diffuses through the tissue space, and eventually
returns to the blood. The extracted fraction depends on the permeability of the membrane to the
water, the flow rate of the labeled water within the
capillary, and the surface area available for transcapillary exchange of the water.
MEASUREMENT OF THE EXTRACTION FRACTION OF WATER
The method we employed to measure the fraction of a labeled substance extracted by the brain
during a single capillary transit used external
detection of a gamma-emitting isotope; this singleinjection method was first proposed by Sejrsen
(19). He demonstrated in isolated cat muscle that
the estimate of the fraction of 51Cr-labeled
ethylenediaminetetraacetic acid (EDTA) extracted
on a single capillary transit determined by this
technique was accurate compared with the estimate
determined by the standard venous sampling technique which uses labeled albumin as the reference
(20). In fact, the accuracy of the single-injection
technique is probably greater than that of the
venous sampling technique because of its experimental simplicity. The perturbing effects of the
venous sampling catheter system are eliminated,
and, since the tracer serves as its own intravascular
reference, there is no Taylor effect (9).
The accuracy of the single-injection technique of
assessing the extraction fraction when it is employed in vivo does require that the peak height of
the radioactivity recorded over the organ of interest
be proportional to the quantity of material injected
and that recirculation of the labeled material be
negligible during the time over which the extrapolation is made. The first requirement was met in
part by the rapid intra-arterial injection of a small
volume (~0.2 ml 0.5 seconds) and the use of short
sampling times (100 msec). Moreover, injections of
labeled water into the internal jugular vein demonstrated that the amount of labeled water recirculating to the monitored tissue during the first 30
seconds was approximately 0.05 (1 - E). Hence,
the overestimation of the extraction fraction due to
recirculation should be limited to about 2%.
MEASUREMENT OF CEREBRAL BLOOD FLOW WITH LABELED
WATER
Our calculation of cerebral blood flow, based on
the central volume principle, employed the mean
brain-blood equilibrium partition coefficient for
water. It is clear from our observations that water is
not in equilibrium with the interior phases of the
brain tissue during the time of our measurement. It
has been argued (21) that under these circumstances it is invalid to employ the equilibrium
value for the partition coefficient. However, Roberts et al. (22) have recently shown in a rigorous
mathematical proof of the central volume principle
that equilibrium between phases as a whole is not a
necessary condition for the valid application of the
central volume principle; only the boundary
between two phases must be in equilibrium. Intuitively it seems paradoxical that TH^ should not
depend on the diffusion coefficients of water in
various phases of brain tissue. Roberts et al. (22)
rationalized their observation by concluding that
the diffusion coefficient and the average depth of
penetration of tracer into the tissue phases are
coupled. In other words, if the diffusion coefficient
is low the average depth of penetration will be
Circulation Retearch, Vol. 35, September 1974
Downloaded from http://circres.ahajournals.org/ by guest on February 23, 2013
362
EICHLING, RAICHLE. GRUBB. TER POGOSSIAN
small, whereas if the diffusion coefficient is high
the penetration depth will be large. The time
required for tracer to enter and then be cleared
from the tissue is, however, the same in both cases,
because the faster diffusing tracer has to travel
correspondingly longer distances.
To test the hypothesis of Roberts et al. (22)
experimentally and to verify our method of computing cerebral blood flow with H215O, we compared computed flow values obtained with H216O
with those obtained with the standard 133Xe 10minute height-over-area technique (23). This comparison was accomplished by sequentially injecting
H 2 ls O and then 133Xe. The time course of each
tracer was monitored and processed as described
earlier in this paper, except that a separate energy
window of acceptance was adjusted symmetrically
around the 81-kev photopeak of 1!3Xe. Suitable
correction was applied to subtract the contribution
of the l5O activity from the 133Xe signal. A value of
1.15 ml/ml or 1.10 ml/g (24) was used for the
equilibrium brain-blood partition coefficient for
133
Xe. Cerebral blood flow was manipulated, as
described earlier, by changing arterial Pco, (see
Methods); measurements of arterial blood gases
bridged each measurement to ensure a steady
state. Thirteen paired observations were made over
the cerebral blood flow range of 20 to 160 ml/100 g
min"'. Cerebral blood flow for l33Xe was calculated
assuming that the 10-minute height-over-area
method systematically overestimates flow by 11%
(23, 25). The resultant line of regression was CBF
(labeled H2O) = 0.97CBF (labeled Xe) + 0.7 with a
correlation coefficient of r = 0.96 (P « 0.001). On
no occasion was a nonextracted fraction observed
in the 133Xe data. The correlation between the two
methods of flow determination, practically one of
identity for the blood flow range of 20 to 160 ml/100
g min" 1 , verifies our method of computing cerebral
blood flow employing H21SO. Furthermore, it provides experimental support for the hypothesis that
equilibrium between the interior of the phases of a
system, in this case the brain, is not a necessary
condition for the valid application of the central
volume principle.
MEASUREMENT OF BRAIN-CAPILLARY WATER PERMEABILITY
Our data provide the opportunity for a more
detailed examination of the brain-capillary
permeability of water. Renkin (26) and Crone (20)
have modeled the loss of diffusible substances from
a single capillary. They assumed that diffusible
material leaving the capillary is promptly sequestered with none of it returning to the capillary and
that the rate of loss of material is proportional to
the remaining concentration at each point. From
these observations they developed the relationship
(5)
Cu =
where P is the capillary permeability, S is the
capillary surface area, Ca and Cu are the concentrations of the material at the arterial and venous ends
of the capillary, respectively, and F is the blood
flow. Crone applied Eq. 5 to data obtained from
whole organs with the implicit assumption that the
transit time through each capillary is the same. To
use the relationship, Crone set the outflow concentration of the reference vascular tracer equal to Ca
and the outflow concentration of the diffusible
tracer equal to Cu and defined the extraction
fraction of the diffusible material E as (Ca Cv)/Ca . Hence, equivalent forms of Eq. 5 can be
expressed as:
1 - E = e~FS/r
(6)
or
l n ( l - E) =
-PS/F.
(7)
Despite its limitations, the single capillary
model of Renkin and Crone can be used for the
calculation of the brain PS (capillary permeability-surface area product) for water from our
data. Figure 4 shows the result of 47 studies in
which the natural logarithm of (1 - E) was plotted
vs. the reciprocal of cerebral blood flow. These data
demonstrate a significant linear relationship (P «
0.001). The equation for the regression line is:
ln(l - E) = 0.04 - 138/CBF,
(8)
with a correlation coefficient of r = -0.97. Thus,
the relationship predicts that the capillary PS in
the brain is approximately 138 ml/100 g min~' or
0.023 cm3/sec g~\
Data on the value of PS for water in the brain are
not, to our knowledge, available in the literature.
The only comparable data are those recently reported by Eckman et al. (27) for the diffusible
indicator antipyrine. Utilizing an autoradiographic
technique, they estimated a regional cerebral cortical capillary PS value for MC-antipyrine of
0.019-0.027 cm'/secg-'.
To express P as the conventional literature
permeability based on indicator concentrations per
milliliter of solvent water on both sides of the
barrier, the cerebral blood flow should be converted
to flow rate of blood water, Fw . The water content
of blood varies only slightly with changing hematoCirculation Research, Vol. 35, September 1974
Downloaded from http://circres.ahajournals.org/ by guest on February 23, 2013
363
DIFFUSION LIMITATIONS OF WATER
100
50
o
o
a.
u- 5
o
2 2
0
.005 .010 .015 .020 .025 .030
I/CBF (irH/IOOg/min)''
e>
.035
FIGURE 4
Natural logarithm of the nonextracted fraction of H,"0 during
a single cerebral passage, ln{l - E), plotted as a function of the
reciprocal of the cerebral blood flow (1/CBF). The equation of
the linear regression line is ln(l - E) = 0.05 - 138/CBF (r =
-037, P « 0.001).
crit and is approximately 0.81 ml blood water/ml
blood for most mammals (28). Thus, Eq. 8 becomes
ln(l - E) = 0.04 - l\2/Fw,
(9)
predicting a value of PS of 0.019 cm'/sec g" 1 .
Assuming an average capillary surface area on
the order of 100 cm'/cm 1 (cerebral cortex [human]
190 cmVcm3 and white matter [human] 57 cm2/
cm3 [29]) permits an estimate of P.
0.019 cmVsec cm- 3
lOOcmVcm3
1.9 x 10"4 cm/sec.
This value can be compared with water permeabilities of 33 x 10"4 cm/sec for the human red
cell membrane (30) and 20 x 10"4 cm/sec for
artificial lipidfilms(31).
The reason brain is apparently less permeable to
water than is myocardium or skeletal muscle is not
clear. Two factors might be responsible. First, the
capillary density in the brain is considerably less
than that in the myocardium (1), thus reducing the
capillary surface area available for exchange. Second, the capillaries in the brain are structurally
unique; they have no apparent openings between
constituent endothelial cells (32).
In conclusion, labeled water cannot be viewed as
a freely diffusible internal standard when the
blood-brain barrier passage of other substances is
being evaluated. At normal cerebral blood flow for
the rhesus monkey (~50 ml/100 g min"1), only
about 90% of the injected bolus of labeled water
freely exchanges with brain; this value decreases
progressively with higher cerebral blood flows. For
smaller animals in which cerebral blood flow is
considerably higher (33), this problem is probably
magnified.
Our findings confirm experimentally the hypothesis that the valid application of the central volume
principle does not depend on the achievement of
tracer equilibrium within the interiors of various
phases of a system (22) but rather only requires
equilibrium at the boundary between phases. As
such this observation justifies the use of Hj ls O for
the measurement of cerebral mean transit time and
cerebral blood flow.
Finally, the feasibility of measuring the fractional extraction of labeled substances during a
single capillary transit in vivo using external detection of a positron-emitting isotope has been demonstrated. This approach provides not only a means
of evaluating the blood-brain permeability to water
under a variety of experimental conditions but also
of evaluating the blood-brain barrier permeability
and transport characteristics of other similarly
labeled substances.
Acknowledgment
The authors wish to thank Mr. Julius Hecht, Mr. Robert A.
Feldhaus, Ms. Gail Batek, and the staff of the Washington
University Medical School cyclotron for their invaluable technical assistance in these experiments. We also thank Dr. K. B.
Larson of the Biomedical Computer Laboratory, Washington
University, for his valuable suggestions concerning data analy-
References
1. KETY SS: Theory and applications of the exchange of inert
gas at the lungs and tissues. Pharmacol Rev 3:1-41,
1951
2. JOHNSON JA, CALVERT HM, LIFSON N: Kinetics concerned
with distribution of isotopic water in isolated perfused
dog heart and skeletal muscle. Am J Physiol 171:687-693,
1952
3. YIPINTSOI T, BASSINCTHWAICHTE J: Circulatory transport of
iodoantipyrine and water in the isolated dog heart. Circ
Res 27:461-477, 1970
4. YuDtLEvrcH DL, DEROSE N: Blood-brain transfer of glucose
and other molecules measured by rapid indicator dilution. Am J Physiol 220:841-846, 1971
5. LIFSON N: Kinetics of distribution of isotopic water in the
perfused dog liver. In Capillary Permeability, edited by C
Crone and N Lassen. New York, Academic Press, 1970,
pp 569-577
6. OLDENDORF WH: Brain uptake of radiolabeled amino acids,
amines, and hexoses after arterial injection. Am J Physiol
221:1629-1639, 1971
Circulation Research, Vol. 35, September 1974
Downloaded from http://circres.ahajournals.org/ by guest on February 23, 2013
364
EICHLING. RAICHLE, GRUBB, TER-POGOSSIAN
7. OLDENDORF WH: Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res 24:372-376, 1970
8. OLDENDORF WH: Blood-brain barrier permeability to lactate. Eur Neurol 6:49-55, 1971
9. LASSEN N, CRONE C: Extraction fraction of a capillary bed to
hydrophilic molecules: Theoretical considerations regarding the single injection technique with a discussion of the
role of diffusion between laminar streams (Taylor's effect). In Capillary Permeability, edited by C Crone and N
Lassen. New York, Academic Press, 1970, pp 48-59
10. TER-POCOSSIAN MM,
EICHLJNG J,
Physiol Scand 58:292-305, 1963
21. BASSINGTHWAIGHTE JB, STRANDELL T, DONALD DE: Estima-
tion of coronary blood flow by washout of diffusible
indicators. Circ Res 23:259-278, 1968
22. ROBERTS GW, LARSON KB, SPAETH EE: Interpretation of
23.
24.
DAVIS D, WELCH M,
METZGER J: Determination of regional cerebral blood flow
by means of water labeled with radioactive oxygen-15.
Radiology 93:31-40, 1969
25.
26.
11. WELCH MJ, LIFTON JF, TER-POGOSSIAN MM: Preparation of
millicurie quantities of oxygen-15 labeled water. J Labeled
Compounds 5:168-172, 1969
12. ZIERLER KL: Equations for measuring blood flow by external monitoring of radioisotopes. Circ Res 16:309-321, 1965
27. ECKMAN WW, PHAIR RD, FENSTERMACHER JD, SOKOLOFF L:
13. SCHMIDT CF, KETY SS, PENNES HH: Gaseous metabolism of
the brain of the monkey. Am J Physiol 143:33-52, 1945
14. PROSENZ P: Investigations on the filter capacity of the dog's
brain. Arch Neurol 26:479-488, 1972
28.
15. HASEGAWA T, RAVENS JR, TOOLE J F : Precapillary arteriove-
29.
nous anastomosis: "Thoroughfare channels" in the brain.
Arch Neurol 16:217-224, 1967
16. ROWBOTHAM GF, LITTLE E: A new concept of the circulation
17.
18.
19.
20.
of the brain: Discovery of surface arteriovenous shunts. Br
J Surg 52:539-542, 1965
SOLNITZKY O: Angio-architecture of the cerebral cortex of
macacus rhesus. Anat Res [Suppl] 76:252-259, 1940
CONLON T, OUTHRED R: Water diffusion permeability of
erythrocytes using a NMR technique. Acta Biochim
Biophys 288:354-361, 1972
SEJRSEN P: Single injection, external registration method for
measurement of capillary extraction. In Capillary
Permeability, edited by C Crone and N Lassen. New
York, Academic Press, 1970, pp 256-260
CRONE C: Permeability of capillaries in various organs as
determined by use of the indicator diffusion method. Acta
mean transit time measurements for multiphase systems.
J Theor Biol 39:447-475, 1973
HOEDT-RASMUSSEN K: Regional cerebral blood flow. Acta
Neurol Scand 43(suppl 27): 1-81, 1967
VEALL N, MALLETT BL: Partition of trace amounts of xenon
between human blood and brain tissues at 37°C. Phys
Med Biol 10:375-380, 1965
LASSEN N: Cerebral blood flow and oxygen consumption in
man. Physiol Rev 39:183-238, 1959
RENKIN EM: Transport of potassium-42 from blood to tissue
in isolated mammalian skeletal muscles. Am J Physiol
197:1205-1210, 1959
30.
31.
Measurement of regional blood-brain barrier permeability to MC-antipyrine in normal cats (abstr). Fed Proc
32:324, 1973
DITTMER D (ed): Blood and Other Body Fluids. Washington,
D. C , Federation of American Societies for Experimental
Biology, 1961, pp 19, 326
COBB S: Cerebrospinal blood vessels. In Penfield's Cytology
and Cellular Pathology of the Nervous System, vol II.
New York, Paul B. Hoeber, Inc., 1932, pp 577-610
SAVITZ D, SOLOMON AK: Tracer determination of human red
cell membrane permeability to small non-electrolytes. J
Gen Physiol 58:259-266, 1971
HARDON D: Diffusion of water through artificial lipid membranes and the influence of unstirred layers. In Capillary
Permeability, edited by C Crone and N Lassen. New
York, Academic Press, 1970, pp 492-499
32. MAYNARD EA, SCHULTZ RL, PEASE DC: Electron microscopy
of the vascular bed of the rat cerebral cortex. Am J Anat
100:409-433, 1957
33. HAINING JL, TURNERMD, PANTALL RM: Local cerebral blood
flow in young and old rats during hypoxia and hypercapnia. Am J Physiol 218:1020-1024, 1970
Circulation Research, Vol. 35. September 1974
Downloaded from http://circres.ahajournals.org/ by guest on February 23, 2013