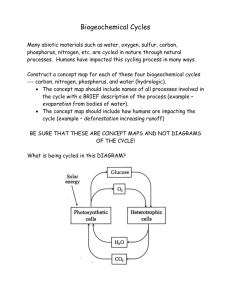
Performance Benchmark E.12.C.3
advertisement

Performance Benchmark E.12.C.3 Students know elements exist in fixed amounts and move through solid earth, oceans, atmosphere and living things as part of biogeochemical cycles. E/S With few exceptions Earth is a closed system with respect to matter. The elements that are present on Earth today are the same elements that were present 4.6 billion years ago. Earth’s processes, driven by energy transfer, provide the mechanisms that allow for the circulation of these elements that exist in relatively fixed quantities. Biogeochemical cycles describe the movement (or cycling) of matter through Earth’s systems. In general the systems can be subdivided, for ease of examination, into the atmosphere, hydrosphere, lithosphere, and biosphere. The movement of matter through and between each subsystem is a biogeochemical cycle. The cycling elements of most importance to humans are: carbon, hydrogen, oxygen, nitrogen, phosphorous, and sulfur. These elements are needed in large quantities. Others needed in large quantities include potassium, calcium, iron, and magnesium. All other elements cycle through Earth’s processes but are of less importance for our immediate survival; including boron (green plants), copper (some enzymes), and molybdenum (nitrogen-fixing bacteria) to name a few. Figure 1. Generalized Biogeochemical Cycle: The major parts of the biosphere are connected by the flow of chemical elements and compounds. In many of these cycles, the biota plays an important role. Matter from the Earth’s interior is released by volcanoes. The atmosphere exchanges some compounds and elements rapidly with the biota and oceans. Exchanges of materials between rocks, soil, and the oceans are generally slower by comparison. (from, http://www.colorado.edu/GeolSci/courses/GEOL1070/chap04/chapter4.html) There are two energy sources on Earth, the Sun and the core, that enable biogeochemical cycles to perpetuate. The Sun is responsible for nearly all movement of organic matter (carbon-carbon bonds). The Sun and Earth’s core are responsible for movement of inorganic matter. Energy flow through the ecosystem describes the impetus for biogeochemical cycling. The diagram below shows the basics of energy flow through an ecosystem. Energy drives the movement of organic matter and inorganic matter (biogeochemical cycles). Figure 2. Energy flows and material cycles through Earth. (from http://www.globalchange.umich.edu/globalchange1/current/lectures/kling/ecosystem/ecosystem.html) The flow of energy as well as organic and inorganic matter through an ecosystem can be generalized by: The ultimate source of energy (for most ecosystems) is the sun. The ultimate fate of energy in ecosystems is for it to be lost as heat. Energy and matter are passed from organism to organism through the food chain as one organism eats another. Decomposers remove the last energy from the remains of organisms. Inorganic matter is cycled, energy is not. To learn more about energy flow through Earth’s systems, go to http://www.globalchange.umich.edu/globalchange1/current/lectures/kling/ecosystem/eco system.html The Carbon Cycle: Carbon is one of the fundamentally important elements to all life. The biogeochemical cycle of carbon is complex in that parts of the cycle can take days to complete while others can take millennia. The carbon cycle can be broken down into two main parts: geological cycling and biological cycling. The four main storage areas of carbon are: as carbon dioxide (CO2) in the atmosphere, as organic compounds in living or recently dead organisms, as dissolved carbon dioxide in the oceans and other bodies of water, and as calcium carbonate in limestone and in buried organic matter (fossil fuels). Figure 3. Volcanic Eruption. (from http://essp.csumb.edu/esse/climate/climatebiogeo.html) Figure 4. Carbon cycle diagram shows amounts of carbon transfer in Gigatons per year. (from http://www.metoffice.gov.uk/research/hadleycentre/models/carbon_cycle/intro_global.html) Carbon dioxide is continuously removed from the atmosphere by plants. Plants, through photosynthesis, make sugars that may be transferred to animals. Upon death most of the carbon, through oxidation, is returned to the atmosphere. A small portion of the carbon may become transferred into the geologic cycle with sedimentary rocks. This accounts for coal and petroleum deposits. There is also an exchange of carbon dioxide between the atmosphere and bodies of water. These exchanges range from short term, as in aquatic plants using CO2 through photosynthetic process and many aquatic animals using CO2 and calcium to make shells of calcium carbonate (CaCO3); to long term when aquatic animals die and their shells accumulate on the ocean floor. The CaCO3 accumulates in mass as sediment on the ocean floor. In time the buried sediment undergoes compaction and cementation to form limestone. Perhaps millennia later to be released back into the atmosphere by the plate tectonic cycle by means of volcanic activity. Carbon also plays a major role in the temperature on Earth. As more CO2 is released from storage areas such as: plant material, fossil fuels, and geologic process (volcanoes), more of the Sun’s energy is reflected back and forth between the surface and the atmosphere of Earth. This increased reflection and absorption of energy affects Earth, likely increasing its temperature. To learn more about the carbon cycle and global warming, go to http://earthobservatory.nasa.gov/Library/CarbonCycle/carbon_cycle.html To learn more about the carbon cycling through biological and geological process, go to http://www.elmhurst.edu/~chm/vchembook/306carbon.html The Water Cycle Figure 5. Hydrologic cycle. (from, http://watercycle.gsfc.nasa.gov/images/watergraphic_low.jpg) The hydrological cycle (water cycle) is important to all living things and is also the major weathering and erosion agent that allows the rock cycle to perpetuate. Most of Earth’s water is stored in the oceans. Incoming solar energy continues evaporation of oceans, winds (also caused by the Sun) disperse the water vapor across the planet. Water condenses over the land where it precipitates as snow, rain sleet, fog, etc. which supports all terrestrial life. Precipitation accumulates on land in streams, rivers, lakes, etc. where it can evaporate back into the atmosphere, runoff back to the ocean, or saturate the soil. From the soil, water can percolate into the groundwater system or be taken up by biotic organisms. Water that enters the ground water system may not reenter the water cycle for some time; water may be contained in aquifers for millennia. Water that is taken by plants and animals will cycle through in days and will, most likely, return to the atmosphere through transpiration (plants) and evaporation (elimination from animals). Of the total amount of water on Earth, at any point in time is stored in: 97.6% Oceans 2.07% Ice and snow (glaciers mostly) 0.28% Groundwater 0.009% Lakes and reservoirs 0.007% Saline Lakes 0.005% Soil moisture 0.005% Biological moisture in plants and animals 0.001% Atmosphere 0.0003% Swamps and marshes 0.0001% Rivers and streams To learn more about the hydrologic cycle, go to http://watercycle.gsfc.nasa.gov/ For a video clip of the water cycle, go to http://gwec.gsfc.nasa.gov/movies/EnergyUncomp640.mpg The Oxygen Cycle: By examining the carbon cycle, you will see that oxygen cycles with carbon because of the nature of their bonding. Oxygen is present in carbon dioxide, in carbohydrates, in water, in rocks and minerals, as a molecule of two oxygen atoms, and three oxygen atoms (ozone) in the atmosphere. Oxygen is a by-product of photosynthesis (oxygen is released during hydrolysis in the light reaction) that is released to the atmosphere. Oxygen is used by both plants and animals during cellular respiration (cells use sugar and O2 to regenerate ATP from ADP). The oxygen concentration of Earth is about 21%, which has not always been the case. All of the oxygen in the atmosphere (O2) is biogenic; it was released from water through photosynthesis by ancient autotrophs. This process is estimated to have taken about 2 billion years; and this is the reason that complex multicellular organisms can exist. Oxygen is the most abundant element (by weight) in the Earth’s crust. Because of oxygen’s availability and high electro-negativity oxygen readily bonds with many other elements to form minerals. Of the seven major mineral groups, five need oxygen. The silicates (most abundant on Earth), carbonates, oxides, and sulfates all need oxygen and cycle oxygen through the rock cycle. Exchanges between atmospheric and geologic oxygen occurs through two major processes. Oxygen is removed from the atmosphere when animals use CO2 to form their shells. This will eventually transfer into the rock cycle as limestone. Oxygen is released into the atmosphere from the rock cycle through volcanic activity and the weathering of rocks and minerals containing oxygen. Figure 6. Oxygen cycle (from: http://telstar.ote.cmu.edu/environ/m3/s4/cycleOxygen.shtml) To learn more about the oxygen cycle, go to http://telstar.ote.cmu.edu/environ/m3/s4/cycleOxygen.shtml The Nitrogen Cycle Nitrogen is the most common element in the atmosphere at about 78%. Nitrogen is also vital for all life on Earth because amino acids (constituent of proteins) and nucleic acids (constituent of DNA) would not exist without nitrogen. In the atmosphere nitrogen exists as a very stable molecule (N2) which is unusable by plants and animals. The process of “fixing” nitrogen so that it can be used by plants and animals is carried out by bacteria. Nitrogen fixing bacteria are specialized in that they can use atmospheric nitrogen (N2) and as a byproduct release ammonia (NH4). Next, nitrite-forming bacteria combine the ammonia with oxygen, forming nitrites (NO2-). Another group of bacteria then converts the nitrites to nitrates (NO3-). Nitrites can be absorbed and used by green plants. In plants the nitrates are reduced to ammonium (NH4+) which is used to build amino acids. Animals receive their needed nitrogen from plants. Nitrogen reenters the atmosphere primarily by the actions of decomposers which break down; dead organisms, leaves that fell off in the winter, skin, hair, urine etc. Decomposers use the nitrates (ammonia and ammonium) and produce a byproduct of nitrogen gas, either N2 or N2O. Figure 7. Nitrogen cycle. (from: http://telstar.ote.cmu.edu/environ/m3/s4/cycleNitro.shtml) To learn more about the nitrogen cycle, go to http://essp.csumb.edu/esse/climate/climatebiogeo.html The Phosphorous Cycle Figure 8. Phosphorus cycle. (from: http://www.enviroliteracy.org/article.php/480.html) Phosphorus is needed by organisms mainly as a compound used in energy-transfer (Adenosine Tri-Phosphate and ADT). Phosphorus, often leached from rocks and minerals, is an important component of soils. Phosphorus does not have an atmospheric form, so it is most often transported by water. Inorganic phosphorus is taken in by plants, incorporated into organic compounds, and moves up the food chain. Phosphorus is returned to the soil and rock cycle through decomposition of waste. To learn more about the phosphorus cycle, go to http://www.marietta.edu/~biol/102/ecosystem.html#ThePhosphorousCycle13 And, http://www.enviroliteracy.org/article.php/480.html The Sulfur Cycle Sulfur is another vital element for all living organisms. Sulfur is a minor but important element in some proteins and an indicator of acid rain. In a droplet of water vapor it may act as regulators of climate change. Sulfur is released into the atmosphere and hydrological cycle by the weathering of rocks and minerals (the sulfides and sulfates class), deep sea vents, and volcanoes. Once sulfur is exposed to the atmosphere it converts to a sulfate (SO4). The sulfate can be taken up by plants where it is converted into organic forms and will move up the food chain. Sulfur returns to the soil through decomposition of organic material. From here sulfur can be returned to the rock cycle or taken up again in the biological cycle. Sulfur is also found in fossil fuels, mixing of the biological and geological cycles. When fossil fuels are burned the sulfur is released into the atmosphere again. When concentrations of sulfur in the atmosphere become too high, chemical reaction occur that produce acid rain (H2SO4). Figure 9. Sulfur cycle. (from http://telstar.ote.cmu.edu/environ/m3/s4/cycleSulfur.shtml) To learn more about the sulfur cycle, go to http://telstar.ote.cmu.edu/environ/m3/s4/cycleSulfur.shtml Performance Benchmark E.12.C.3 Students know elements exist in fixed amounts and move through solid earth, oceans, atmosphere and living things as part of biogeochemical cycles. E/S Common misconceptions associate with this benchmark 1. Students often have difficulty with charts and flow diagrams that explain biogeochemical cycles resulting in misinterpreted meaning. In order to effectively address this misconception, students need to be given many examples of biogeochemical cycles with guided practice in interpreting directionality of moving matter. Interactive Biogeochemical Cycle lesson plan that identifies and addresses this issue, http://microbes.arc.nasa.gov/download/pdf/Interactive_Bio_Cycles_Plan.pdf 2. Students incorrectly think that the original substance vanishes "completely and forever" in a chemical reaction. Perhaps out of sight out of mind. Students need constant reinforcement in the law of conservation of matter. They need to be reminded that Earth is a relatively closed system, and that matter, as in a chemical reaction or cycle, is neither created nor destroyed. To see this and other chemistry misconceptions, go to http://educ.queensu.ca/~science/main/concept/chem/c07/C07CDTL1.htm 3. Students incorrectly think of energy as a type or form of matter. The belief that energy can be recycled through an ecosystem is often times confused with the fact that matter is recycled through an ecosystem. Students must be made aware that Earth receives energy from the Sun and the core only. Also, the ultimate fate of energy in ecosystems is for it to be lost as heat. Energy is not recycled, only transferred through an ecosystem to eventually dissipate into space. To view this misconception and many other biological misconceptions, go to http://departments.weber.edu/sciencecenter/biology%20misconceptions.htm 4. Students incorrectly believe that the same water goes around the water cycle forever. To continue with this, the water they drink is the same water dinosaurs drank millions of years ago. Students must be aware of the vastness of the hydrological cycle, and that plants, through photosynthesis, and cells through cellular respiration split water molecules into hydrogen and oxygen (hydrolysis). Most simply stated: The atoms are the same, but the molecule is not. To view this misconception and many other biological misconceptions, go to http://departments.weber.edu/sciencecenter/biology%20misconceptions.htm Performance Benchmark E.12.C.3 Students know elements exist in fixed amounts and move through solid earth, oceans, atmosphere and living things as part of biogeochemical cycles. E/S Sample Test Questions 1. What is the primary source of energy driving the hydrological cycle? a. Earth’s core b. Sun c. Fossil fuels d. Earth’s core and the Sun 2. Why is Earth said to be a closed system to matter and an open system to energy? a. Matter does not cycle through Earth and energy does. b. Energy enters Earth freely and matter can not enter Earth. c. Energy easily enters and leaves Earth, but matter typically does not. d. Matter cycles through Earth, but energy does not. 3. Carbon cycles through atmosphere, biosphere, hydrosphere, and lithosphere (crust). Once carbon is trapped in the lithosphere which of the following is a way in which it can be released directly into the atmosphere? a. Volcanic eruptions b. Carbon fixing bacteria c. Physical weathering of rock d. Evaporation 4. Of the carbon, oxygen, phosphorus and sulfur cycles; which cycle does NOT have an atmospheric component? a. Carbon b. Oxygen c. Phosphorus d. Sulfur 5. Is the water on Earth today the same water that was here 100 million years ago? a. No, water enters and leaves Earth during evaporation and precipitation cycles. b. No, the water present on Earth is made of the same atoms but the molecules have been recycled through biologic activity. c. No, the atoms that made the water then were destroyed when they were used; the atoms that make up water now were made more recently. d. Yes, the molecules of water then are the same molecules that exist today. 6. Use the diagram below to answer the following question. In what two ways can Nitrogen be removed from the atmosphere and cycled through the biological process? a. Lightning and Nitrogen fixing bacteria b. Denitrifying bacteria and Cellular respiration. c. Lightning and Denitrifying bacteria d. Nitrogen fixing bacteria and Denitrifying bacteria Figure reference: http://telstar.ote.cmu.edu/environ/m3/s4/cycleNitro.shtml Performance Benchmark E.12.C.3 Students know elements exist in fixed amounts and move through solid earth, oceans, atmosphere and living things as part of biogeochemical cycles. E/S Answers to Sample Test Questions 1. (b) 2. (c) 3. (a) 4. (c) 5. (b) 6. (a) Performance Benchmark E.12.C.3 Students know elements exist in fixed amounts and move through solid earth, oceans, atmosphere and living things as part of biogeochemical cycles. E/S Intervention Strategies and Resources The following is a list of intervention strategies and resources that will facilitate student understanding of this benchmark. 1. BioEd Online, Biology Teacher Resources from Baylor College of Medicine A great lesson that goes through the water cycle and global warming. “Students will trace the flow of water in the environment. Students will investigate simulated effects of global temperature change on oceanic surface levels. Students will evaluate consequences of changes within water cycle using data from current models. Students will observe and explain several different properties of water.” The lesson takes two days (55 min. each), has prepared slides for a presentation and activities. To learn more about this lesson plan, go to http://www.bioedonline.org/lessons/water-cycle.cfm#audience 2. Environmental Protection Agency (EPA) This site allows students to interact with a water cycle, carbon cycle, and global warming. Students can select a path and follow instruction that allow them to become more familiar with the cycles. This would be great to use as a demonstration in class or as a review of concepts in a computer lab. For access and more information, go to http://epa.gov/climatechange/kids/version2.html. 3. National Center for Atmospheric Research – Traveling Nitrogen Lesson This lesson lets “Students play the role of nitrogen atoms traveling through the nitrogen cycle to gain understanding of the varied pathways through the cycle and the relevance of nitrogen to living things.” This would be a great way to introduce the nitrogen cycle, or use as reinforcement after instruction. To learn more about this lesson, go to http://www.eo.ucar.edu/educators/ClimateDiscovery/ESS_lesson2_10.19.05.pdf 4. NASA has a unit plan on the carbon cycle. “Students review the Geology Training module to observe how Earth’s geology affects carbon dioxide levels. Students create a diagram of the carbon cycle and conclude that the carbon cycle maintains a balance of carbon dioxide in Earth’s atmosphere, thus helping to maintain a moderate surface temperature. They then create and act out two skits of how the carbon cycle works and what would happen if carbon were not released into the atmosphere.” This site links surface temperatures (global warming) and the carbon cycle. To learn more about this unit, go to http://quest.arc.nasa.gov/projects/astrobiology/astroventure/teachers/pdf/AVGeolesson-6.pdf 5. National Science Foundation – Introductory Lesson on Biogeochemical Cycles Through this lesson, “The learner will demonstrate comprehension of the energy sources of various cycles by completing mini stories. The learner will demonstrate analysis of words by defining individual word parts and combining them to form definitions. The learner will demonstrate synthesis of a cycle by researching the cycle and creating a cartoon depicting that cycle.” This is a lesson that may be used as an introduction or a closing review of biogeochemical cycles. For more information about this lesson, go to http://www.envsci.rutgers.edu/~phelps/lessons/lesson3.pdf 6. Environmental Literacy Council This site contains a wealth of information for both educators and students. Links to the major biogeochemical cycles, further reading, and resources for educators are provided. To learn more about their site or to follow their links to lesson plans on cycles, go to http://www.enviroliteracy.org/subcategory.php/198.html.