Spectroscopy
advertisement
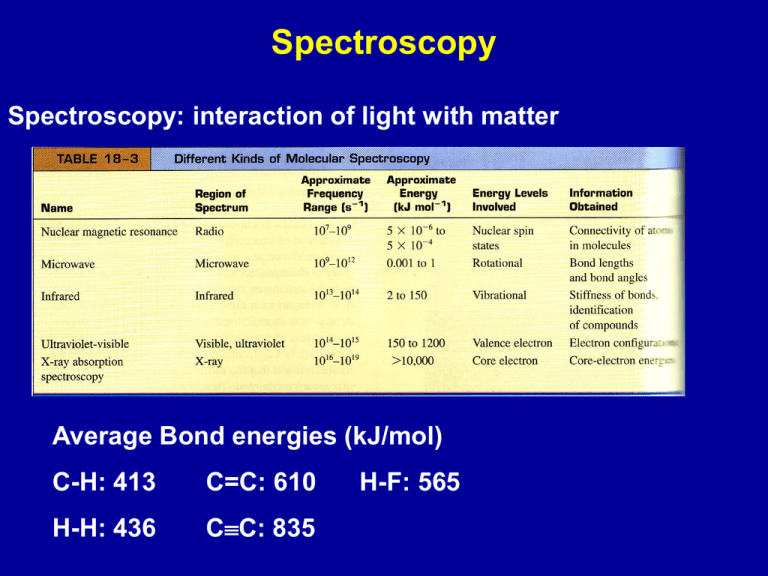
Spectroscopy Spectroscopy: interaction of light with matter Average Bond energies (kJ/mol) C-H: 413 C=C: 610 H-H: 436 CC: 835 H-F: 565 Electronic excitation: visible, uv Vibrational excitation: infrared Rotational excitation: microwave Electronic transitions - visible/ultraviolet Absorption - energy absorbed can excite electrons chlorophyll a chlorophyll b Color of compound - complimentary to wavelengths absorbed Absorption Energy to excite electrons depends on relative position of molecular orbitals If DE is larger - absorption of short wavelength Absorption spectrum of ozone (O3) Emission - molecules in excited electronic states can loose energy by emitting light Chemiluminiscence in fireflies Vibrational excitation: infrared Model the bond between two atoms as a “spring” attached to the two atoms; the spring can vibrate. IR Tutor Units in IR Wavelength m = 10-6 m wavenumber 1 Units cm-1 Organic Chemistry Chemistry of carbon-containing molecules Carbon: has four electrons and four valence orbitals, and so its compounds have neither too few electrons, requiring electron deficient structures, nor too many electrons resulting in excessive lone pair-lone pair repulsions can form compounds containing stable C-C bonds can form stable bonds with a number of elements Result: almost an endless range of compounds with elements bonded to C in straight chains, rings, branched chains and with single, double and triple bonds Hydrocarbons Saturated hydrocarbons: all C - C bonds are single bonds Unsaturated hydrocarbons: C=C or CC bonds Aliphatic hydrocarbons - no benzene ring Aromatic hydrocarbon - contains benzene ring naphthalene Alkanes Saturated hydrocarbons General formula: CnH2n+2 Methane (CH4) Butane (C4H10) and larger alkanes: linear or branched Isomers: same number of atoms, different arrangement n-butane iso-butane Alkanes are considered to be non-polar since the electronegativities of C and H are similar Interaction between hydrocarbon molecules are due to London dispersion forces Strength of these interactions increase with the number of electrons; determines melting and boiling points