Functional Groups
advertisement
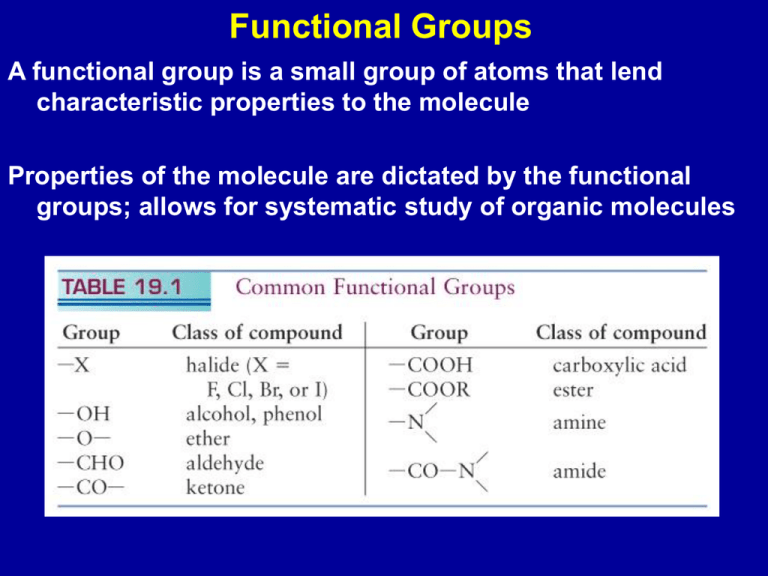
Functional Groups A functional group is a small group of atoms that lend characteristic properties to the molecule Properties of the molecule are dictated by the functional groups; allows for systematic study of organic molecules Halides: R-X where R is any alkyl group, and X a halogen CH3Cl - chloromethane C6H5Cl - chlorobenzene Cl Synthesis: radical chain reaction between an alkane and a halogen CH4 + Cl2 Cl2 hn or heat hn or heat CH3Cl + HCl 2Cl• •Cl + CH4 HCl + •CH3 •CH3 + Cl2 CH3Cl + •Cl Alcohols: R-OH hydroxyl functional group Nomenclature - use the suffix “ol” CH3-CH2-OH ethanol CH3-CH2-CH2OH 1-propanol H 3C C H2 CH2 C H2 OH 1-butanol (1o alcohol) Primary alcohol OH H 3C C H2 CH CH3 2-butanol (2o alcohol) Secondary alcohol OH H 3C C CH3 CH3 2-methyl-2-propanol (3o alcohol) Tertiary alcohol Presence of OH group allows hydrogen bonding As the C chain becomes longer the OH group becomes less important Charge distribution in ethanol; red indicates negative charge Synthesis of alcohols Hydrolysis of a alkyl halide with a strong base OH- + CH3Br CH3OH + BrAddition across a double bond CH2=CH2 + H2O 300 - 400oC 60 - 70 atm CH3CH2OH Phenols: hydroxyl group attached directly to an aromatic ring OH Phenol (C6H5OH) weak acid, Ka = 1 x 10-10; stability of the phenolate ion (C6H5O-) Oil of thyme Oil of clove Ethers: R - O - R C2H5 - O - C2H5 C6H5 - O - C2H5 Synthesis: H SO 2 R-OH 2 4 diethylether ethylphenylether R - O - R + H2O Kinetics vs thermodynamics CH3-CH2-OH + CH3-CH2-OH H2SO4 H2SO4 140oC 180oC 2CH2=CH2 + 2 H2O CH3-CH2-O-CH2-CH3 + H2O Aldehydes and Ketones C O carbonyl group C O aldehyde C O H R R ketone R H HCHO: formaldehyde C O H H3 C CH3CHO: acetaldehyde C O H H3 C CH3COCH3: dimethlyketone (acetone) C H3 C O Properties of aldehydes and ketones differ because of the aldehyde H atom CH3CH2OH 1o alcohol ethanol (CH3)2CHOH 2o alcohol 2-propanol O2, catalyst, CH3CHO aldehyde acetaldehyde high temperatures O2, catalyst, high temperatures (CH3)2CO dimethylketone almond and cherries vanilla cinnamon Carboxylic acids: -COOH functional group O C OH O HCOOH - formic acid (ant venom) H C OH O CH3COOH - acetic acid (vinegar) H3C C OH Carboxylic acids hydrogen bond - “dimers” Synthesis Catalytic oxidation of aldehydes CH3CHO O2, Mn2+ CH3COOH R-COO- R’ Esters: O R C O R' O O H3C C CH2CH3 OH H O acetic acid + ethanol H3C + C OCH2CH3 ethylacetate H2O Fats (solids) and oils (liquids) are triesters formed from glycerol and three carboxylic acids (fatty acids) Tristearin: animal fatty acid tri-esters CH2OHCHOHCH2OH + 3 CH3(CH2)16COOH glycerol stearic acid Saturated - C-C single bonds Unsaturated - one (mono-unsaturated) or more (polyunsaturated) C=C bonds Amines: N H H H N H R primary amine (1o) H N H R secondary amine (2o) R' N R ammonia R' R" tertiary amine (3o) CH3NH2 (CH3)2NH (CH3)3N C6H5NH2 methylamine dimethylamine trimethylamine NH2 aniline N Amines are bases: NicH2+(aq) + 2 NH3(aq) Nic(aq) + 2 NH4+(aq) Synthesis of amines a) Naturally occurring b) NH3 + CH3Cl CH3NH2 + HCl CH3 N nicotine Amino acids: carboxylic acid containing an amine group Glycine: NH2CH2COOH + H3NCHCOOH R cationic form predominant in acidic solutions - H3O+ + H3O+ + H3NCHCOO R dipolar form zwitterion - H3O+ + H3O+ H2NCHCOO R anionic form predominant in basic solutions - R' Amides: -CONH2- group C N O R H Formed by reaction between NH3 or 1o or 2o amine and acid O H3 C O CH2CH3 C O H H NH H3 C C + H 2O NHCH2CH3 O H C HO C H2 N + H HO O O O C C H2 NH2 C HO C H2 N H C C H2 NH2 + H2O glycine + glycine diglycine + H2O Proteins: polypeptides with CONH linkage between amino acids http://www.cryst.bbk.ac.uk/pps97/course/section3/helix.pdb


