Properties Important in Biology
advertisement

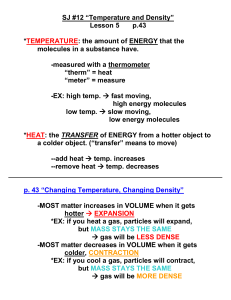
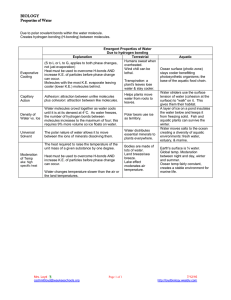
Properties Important in Biology Life and Evolution Cells:70-95% Earth: 3/4 Three states Ecosystems Hydrogen bonds intermolecular Remember charges for each side-unequal distribution Electronegativity of oxygen attracts the positive hydrogen Transient Earth is fit for Life Water sticks to Water ◦ Capillary action Sand and Soil Transpiration ◦ Surface tension Water sticks to other surfaces adhesion Transpiration Capillary Action Water to other molecules Capillary action returns Applications become important for athletes! Water resists temp changes Absorbs heat from air that is warmer Releases heat to air that is cooler Heat reservoir! Oceans have relatively stable temp/Coastal areas stable Marine environment is stable Heat passes from warmer to cooler Ice absorbs liquid heat Water resists temperature changes Heat that a liquid absorbs to go liquid to gas Moderates climates as oceans evaporate and then condenses to form rain Weather patterns Hottest molecules leave, the surface liquid left behind is cooler Contributes to lake and pond temp. and prevents terrestrial life from overheating Expansion upon freezing Ponds and lakesthermal mixing Temp drop 4000/expansionmolecules stop moving Floating ice insulates life below as it releases heat Water gathers around polar molecules Each molecule in cloud of water ◦ Sugar ◦ Salt Sodium + Chloride Alternating ions are held together by electrical charges Water forms hydrogen bonds with cellulose of cotton Towels dry you off- water pulled into the towel Solvent Solute Hydrophilic (ionic and polar molecules/hydrophobic (nonpolar molecules) Biological Rx’s involve solutes dissolved in water Plasma- 90% water Cells- 75-95% water Biological solutions maintain pH because of buffers Acids: increase H+ Bases: reduces H+ Buffers Light Penetrates ◦ Tissues ◦ Aquatic environments Fossil fuel burning creates sulfer oxides and nitrous oxides+ water=strong acids in rain Coal production more oxides+wind= carry acids for miles as rain Fossil fuel burning creates carbon dioxide ◦ Reflective blanket preventing heat escape ◦ Absorbed by oceans