1. Introduction
advertisement

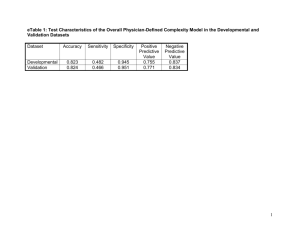
1. Introduction The bioactivity of implantable biomaterials can be strengthened by the formation of biologically active bone-like apatite coatings on its surface. Several methods have been used to prepare such apatite coatings on implantable biomaterials [1], [2], [3], [4] and [5]. Among them, a biomimetic process, which enables the formation of a dense and uniform layer of bone-like apatite on various kinds of materials in different shapes, has been widely used. In this process, the substrate was soaked in a simulated body fluid (SBF) with ion concentrations nearly equal to those of human body plasma at 36.5 °C [6]. The use of the biomimetic method to form an apatite layer on implantable materials was very attractive because of its low formation temperature, uniform thickness, excellent adhesion and inexpensive production cost. To date, apatite coatings have been prepared on a large number of materials including surface-modified silk fabrics [7], surface-modified cotton [8], bioglass [9], silica gel [10], Titanium [11], Tantalum [12], polyamide films [13], CM-chitin and gellan gum gels [14], Polyethyleneterephthalate (PET) [15], and polytetrafluoroethylene (PTFE) membranes [16] by this biomimetic process. In view of the widespread use of the biomimetic process, it is rather surprising that the literature documents seldom reported the formation of apatite coatings on conducting polymers by this method. Polypyrrole (PPy) is one of the most frequently investigated conducting polymers for its good stability and high conductivity. In recent years, the biomedical applications of PPy have been reported because of its good biocompatibility with the mammalian cells and the ability to modify cellular activities by electrical stimulation [17], [18], [19] and [20]. Therefore, PPy and its composite might be used as the next generation implantable biomaterials, which will be capable of interactive and programmable, and thus capable of seamless communication with surrounding tissues, to regulate cell attachment, proliferation, and differentiation through the electrical stimulation. So it would be very attractive to incorporate electroactive PPy with the bone-like apatite to prepare a novel bioactive composite which would be a desirable candidate for the coatings of the metal implants and tissue engineering scaffolds due to its ability to bond to bone as well as to affect cellular activities through electrical current or field. In the present paper, we show that a thin bone-like apatite layer can be fabricated on PPy substrate doped with heparin sodium and P-TSA by the biomimetic process using SBF. The result means that PPy itself, when doped by some chemical substances containing special functional groups, may exhibit bone-bonding ability, i.e. bioactivity. This work serves as an important first step toward the development of PPy based biomimetic implantable composite with enhanced tissue–implant interactions. In order to reveal the fundamental conditions for obtaining apatite/PPy composite materials using biomimetic method, the preparation of the apatite coatings on PPy, which formed with and without dopants, were investigated. The possible mechanism responsible for the formation of apatite coatings was also discussed. 2. Experimental Pyrrole monomer was purified by distillation under nitrogen atmosphere before use, stored at low temperature and protected from light. All other reagents were of analytical grade and were used as received. De-ionized water was used throughout this work. Pyrrole (0.15 ml dissolve in 20 ml de-ionized water), P-TSA (0.30 g), and heparin sodium salt (0.05 g) were dissolved in 20 ml de-ionized water and stirred magnetically in the ice bath. Then an aqueous solution of (NH4)2S2O8 (0.80 g dissolve in 20 ml de-ionized water) was added to the above solution slowly (0.5 ml/min). The mixture was stirred for another 10 min to ensure complete mixing. Then the polymerization was allowed to proceed without agitation for 24 h at ice bath. PPy doped with heparin sodium salt and P-TSA was obtained after filtration and rinsing with de-ionized water for several times and dried at 80 °C overnight. Finally, as-obtained PPy was compressed into pellets of 15 mm diameter and about 1mm in thickness. Meanwhile, PPy also synthesized without dopants through the same process for a comparative study. The obtained PPy pellets were soaked in 30 ml of SBF, which was prepared by dissolving appropriate quantities of the reagent-grade chemicals of NaCl, NaHCO3, KCl, K2HPO4·3H2O, MgCl2·6H2O, CaCl2 and Na2SO4 in de-ionized water and buffered at 7.40 using tris-hydroxymethylaminomethane ((CH2OH)3CNH2) and hydrochloric acid at 36.5 °C [18], for a predetermined periods. Then the samples were removed from the solution, washed with de-ionized water and dried at room temperature. X-ray diffraction (XRD) and energy-dispersive X-ray analysis (EDX) were used to characterize the elemental composition and surface chemistry of the SBF-immersed samples. The surface morphology of the specimens was observed by a scanning electron microscopy (SEM, LEO1530). 3. Results and discussions Fig. 1 shows SEM photographs of the surfaces of doped PPy samples after soaking in SBF for 0, 7, and 21 days. It can be seen clearly from these pictures that particles were formed on the doped PPy. This particle morphology is similar to that of apatite crystals formed on polyamide films after having been soaked in SBF [21]. The EDX results distinctly showed that the particles contained predominantly calcium and phosphorus (Fig. 2). (114K) Fig. 1. SEM images of the surfaces of doped PPy before and after soaking in SBF for 7 and 21 days. Doped PPy without soaking is denoted as “0 d”. (18K) Fig. 2. EDX spectra of doped PPy after soaking in SBF for 7 and 21 days. Fig. 3 shows XRD patterns of the surfaces of doped PPy before and after immersion in SBF for 7 and 21 days. XRD peaks were observed at 2θ = 26°and 32° after soaking and these peaks were all ascribed to a crystalline apatite, indicating that the particles formed on doped PPy in Fig. 1 are apatite. (4K) Fig. 3. XRD patterns of doped PPy before and after soaking in SBF for 7 and 21 days. Doped PPy without soaking is denoted as “0 d”. Fig. 4 shows the SEM photographs of the surface of undoped PPy before and after soaking in SBF for 21 days. As shown in Fig. 4, there is no obvious difference between the morphologies of undoped PPy even after soaking in SBF for up to 21 days, which means that apatite could hardly formed on undoped PPy. Therefore, we considered that the dopants might play an important role in the formation of the apatite layers on the PPy substrate. (91K) Fig. 4. SEM images of the surfaces of PPy before and after soaking in SBF for 21 days. PPy without soaking is denoted as “0 d”. It has been reported that some organic polymers containing negatively charged functional groups, such as carboxyl groups [22], sulfonic groups [21] and phosphate groups [16] have ability to induce deposition of apatite on their surfaces in SBF. Thus, it is reasonable to postulate that the apatite layer formed on doped PPy are induced by the heparin sodium salt and P-TSA which contained carboxyl groups ( COOH) and sulfonic groups ( SO3H) and been used as dopants in the synthesis of PPy. Under the induction of these dopants, apatite nuclei were formed and spontaneously grew to apatite crystal by consuming the calcium and phosphate ions from the surrounding fluid. 4. Conclusions Apatite coatings were fabricated on the surface of PPy doped with heparin sodium and P-TSA by a biomimetic process utilizing SBF. We propose that the formation of apatite depended on the presence of carboxyl groups and sulfonic groups contained dopants. The resulting apatite/PPy composite may have great potential for the development of conducting polymers based bioactive coatings on metal implants and tissue engineering scaffolds. We are currently exploring the factors affecting the formation of apatite coatings in order to increase the content of apatite in the apatite/PPy composite within a relatively short period. Acknowledgements This work was supported by the Research Foundation of Xiamen University (No. 2003xdyy28). References [1] M. Stewart, J.F. Welter and V.M. Goldberg, J. Biomed. Mater. Res. 69 (2004), pp. A1–A10. [2] C.C. Almeida, L.A. Sena, A.M. Rossi, M. Pinto, C.A. Muller and G.A. Soares, Key. Eng. Mat. 254 (2004), pp. 729–732. Abstract-Compendex [3] H.B. Hu, C.J. Lin, P.P.Y. Lui and Y. Leng, J. Biomed. Mater. Res. 65 (2003), pp. A24–A29. [4] S. Hirai, K. Nishinaka, K. Shimakage, M. Uo and F. Watari, J. Am. Ceram. Soc. 87 (2004), pp. 29–34. Abstract-INSPEC | Abstract-Compendex [5] W.H. Song, Y.K. Jun, Y. Han and S.H. Hong, Biomaterials 25 (2004), pp. 3341–3349. SummaryPlus | Full Text + Links | PDF (513 K) [6] T. Kokubo, H. Kushitani, S. Sakka, T. Kitsugi and T. Yamamuro, J. Biomed. Mater. Res. 247 (1990), pp. 21–34. [7] Y. Tamada, T. Furuzono, T. Taguchi, A. Kishida and M. Akashi, J. Biomater. Sci. Polymer. Edn. 10 (1999), pp. 787–793. Abstract-MEDLINE | Abstract-EMBASE [8] M.R. Mucalo, Y. Yokogawa, T. Suzuki, Y. Kawamoto, F. Nagata and K. Nishizawa, J. Mater. Sci. Mater. Med. 6 (1995), pp. 658–669. Abstract-Compendex | Abstract-EMBASE | Full Text via CrossRef [9] L.L. Hench and O. Andersson, Intro. Bioceramics (1993), pp. 41–62. [10] P. Li, C. Ohtsuki, T. Kokubo, K. Nakanishi, N. Soga and K. Degroot, J. Biomed. Mater. Res. 28 (1994), pp. 7–15. Abstract-MEDLINE | Abstract-EMBASE | Abstract-Compendex | Full Text via CrossRef [11] T. kokubo, H.M. Kim, M. kawashita and T. Nakamura, J. Mater. Sci. Mater. Med. 15 (2004), pp. 99–107. Abstract-INSPEC | Abstract-Compendex | Abstract-EMBASE | Abstract-MEDLINE | Full Text via CrossRef [12] T. Miyazaki, H.M. Kim, T. Kokubo, C. Ohtsuki, H. Kato and T. Nakamura, Biomaterials 23 (2002), pp. 827–832. SummaryPlus | Full Text + Links | PDF (272 K) [13] T. Kawai, C. Ohtsuki, M. Kamitakahara, T. Miyazaki, M. Tanihara, Y. Sakaguchi and S. Konagaya, Biomaterials 25 (2004), pp. 4529–4534. SummaryPlus | Full Text + Links | PDF (570 K) [14] M. Kawashita, M. Nakao, M. Minoda, H.M. Kim, T. Beppu, T. Miyamoto, T. Kokubo and T. Nakamura, Biomaterials 24 (2003), pp. 2477–2484. SummaryPlus | Full Text + Links | PDF (352 K) [15] H.M. Kim, K. Kishimoto, F. Miyaji, T. Kokubo, T. Yao, Y. Suetsugu, J. Tanaka and T. Nakamura, J. Biomed. Mater. Res. 46 (1999), pp. 228–235. Abstract-MEDLINE | Abstract-EMBASE | Full Text via CrossRef [16] L. Grondahl, F. Cardona, K. Chiem, E. Wentrup-Byrne and T. Bostrom, J. Mater. Sci. Mater. Med. 14 (2003), pp. 503–510. Abstract-Compendex Full Text via CrossRef | [17] B. Garner, A. Georgevich, A.J. Hodgson, L. Liu and G.G. Wallace, J. Biomed. Mater. Res. 44 (1999), pp. 121–129. Abstract-MEDLINE | Abstract-EMBASE | Abstract-Compendex | Full Text via CrossRef [18] J.H. Collier, J.P. Camp, T.W. Hudson and C.E. Schmidt, J. Biomed. Mater. Res. 50 (2000), pp. 74–84. [19] E.D.E. Giglio, L. Sabbatini, S. Colucci and G. Zambonin, J. Biomater. Sci. Polym. Edn. 11 (2000), pp. 1073–1083. [20] H. Castano, E.A. O’Rear, P.S. McFetridge and V.I. Sikavitsas, Macromol. Biosci. 4 (2004), pp. 785–794. Abstract-EMBASE | Abstract-Compendex | Abstract-Elsevier BIOBASE | Abstract-MEDLINE | Full Text via CrossRef [21] T. Kawai, C. Ohtsuki, M. Kamitakahara, T. Miyazaki, M. Tanihara, Y. Sakaguchi and S. Konagaya, Biomaterials 25 (2004), pp. 4529–4534. SummaryPlus | Full Text + Links | PDF (570 K) [22] M. Kawashita, M. Nakao, M. Minoda, H.M. Kim, T. Beppu, T. Miyamoto, T. Kokubo and T. Nakamura, Biomaterials 24 (2003), pp. 2477–2484. SummaryPlus | Full Text + Links | PDF (352 K)