Type of Bond Force holding atoms or ions electronegativity
advertisement

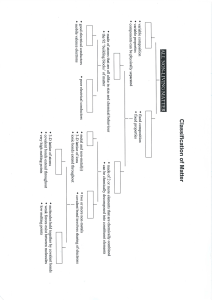
Type of Bond Force holding atoms or ions together electronegativity difference properties particles involved in bonding: Ions Ionic electrostatic attraction ≥ 1.67 stronger than covalent high melting points usually soluble in H2O only conduct molten/dissolved in H2O solids: form crystals weaker than ionic low melting points poor conductors brittle luster (shiny) malleable/ductile good conductors in all states don’t form normal covalent bonds group 1: soft group2: in between transition: hard Transfer of e- (give/take) particles: neutral atoms Polar Covalent unequal sharing 0.2 to 1.67 overlap of e- clouds Covalent Sharing e- Non-polar Covalent equal sharing ≤ 0.2 delocalized electrons Metallic N/A