Synapse formation during development BCS 249 Lecture 14 March 21, 2016

Synapse formation during development
BCS 249 Lecture 14
March 21, 2016
The nervous system: originally thought to be a continuous network
Early action potentials are slow and require Ca 2+ channels
Xenopus
Rohon-Beard neurons
Ca 2+ channels
Na + /Ca 2+ channels
Na + channels
Developmental regulation of Ca 2+ channels T-type vs. L- & N-type
DiI transported by motor neurons
T-type: low voltageactivated
N-, L-type: high voltage-activated
Target-derived retrograde signal from iris & afferent-derived signal from OMN turns on Ca 2+ -dep K + channels
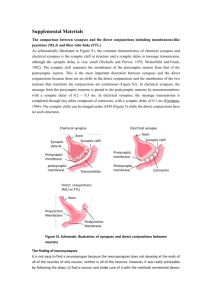
Mature synapse: presynaptic vesicles + postsynaptic density
EB: auditory nerve endbulb; SBC: spherical bushy cell
& myopodia
The stepwise birth of a synapse
Lonely half-synapses prepare for finding a partner
Fig. 8.9 Pre- and postsynaptic differentiation without a partner. A. An electron micrograph showing clustering of α2 adrenergic receptor (arrows) in a postnatal day 4 rat visual cortex neuron. Both of the red-tinted structures are dendrites. B. An electron micrograph showing an apparent presynaptic terminal adjacent to hemolymph. This terminal is made by a Drosophila motoneuron in a mutant strain, twisted ( twi ), that does not generate postsynaptic muscle cells.
Peak synaptogenesis is postnatal & overlaps w/ rapid glial & vascular development
Volume expansion
Peak period of synaptic growth
Growth cones release neurotransmitter (eg. ACh)
Growth cone filopodia can release synaptic vesicles
Dye uptake assay: detect SV release
Fig. 8.12 A presynaptic vesicular release mechanism is present in growth cone filopodia. A. A schematic of the FM dye technique. The dye is taken up into vesicles as they fuse with the membrane. When the terminal depolarized again, the vesicles fuse with the membrane again and the terminal unloads the dye. B. Release of FM4 –64 from growth cone filopodia in response to depolarization (yellow arrows). C. Colocalization of
FM4 –64 (red) and the synaptic vesicle protein, synaptophysin (green), in growth cone filopodia.
Nerve-muscle contact elicits rapid synaptic transmission
“myoball”
Xenopus spinal neurons
postsynaptic currents synaptic transmission triggering an action potential
Netrin depolarizes growth cone, Sema3A hyperpolarizes; target cell contact increases intracellular Ca 2+
Rapid adhesion at the neuromuscular junction synapse
Cadherins/catenin and Nectins/afadin: synCAMs tethered to cytoskeleton
Presynaptic transport packets deliver synaptic proteins to new synapses
A) VAMP-GFP added to neuron
B) FM4-64 labels new presynaptic active zones
C) Syn-RFP colocalizes w/
PSD-95 or
Nlgn
Syn: presynaptic marker
PSD-95, Nlgn: postsynaptic proteins
Receptor-ligand interactions between growth cone and target promote presynaptic maturation pontine neurons: relay signals from motor cx to pons
Fig. 8.18 Signals that promote differentiation of growth cones into presynaptic boutons. A. When imaged continuously in dissociated culture, pontine growth cones (arrows) were observed to stop when they contacted granule cells (arrowhead), their normal postsynaptic target. B. Pontine growth cones express three receptors, Frizzled, FGFR2, and neurexin, on their surface. Granule cells express their respective ligands: Wnt7a, FGF22, and Neuroligin. These signaling pathways recruit vesicles and synaptic proteins to the site of contact.
Presynaptic formation in C. elegans involves signals from a epithelial or glial cell
Syg-1/Syg-2:
Ig superfamily
Unc-6: Netrin
Unc-40: NetrinR
Fig. 8.19 Induction of presynaptic differentiation in C. elegans . A. HSN neurons control egg-laying by forming synapses onto the vulva muscles and the VC4 and 5 interneurons. The presynaptic specializations (box, red rectangle) form opposite the point where epithelial cells contact HSN. B. The signaling cascade that leads to presynaptic induction in HSN axons has been identified through genetic screens. The epithelial cells express an immunoglobulin superfamily member (SYG-2) that recruits a transmembrane molecule expressed by HSN cells (SYG-1) to the position where a presynaptic site will form. This signaling system recruits 2 active zone proteins (SYD-1 and -2) that are key regulators of presynaptic assembly and stability.
Elsewhere in the organism, glial cells release UNC-6 (also known as Netrin in vertebrates), which activates its receptor
(UNC-40) to promote the assembly of a presynaptic specialization.
AChRs can cluster on muscle fibers without innervation
Immature AChR clustering is tightened and refined after innervation
Identification of a matrix proteoglycan, agrin, that promotes
AChR clustering adult frog cutaneous pectoral muscle
Agrin’s receptor is Lrp4, and MuSK & rapsyn also promote clustering blue: MuSK phos’n red: AChR phos’n green: AChR clustering
EphB promotes NMDAR and AMPAR clustering in the CNS cortical neurons transfected w/
EphB2-GFP, exposed to ephrinB1
HI neurons transfected w/
GFP & stained for AMPAR (red)
Pulses of Glu allow mapping of dendritic GluRs by PSPs
Fig. 8.25 Mapping glutamate receptor location during synaptogenesis. A. To map the location of glutamate receptors, an iontophoretic pipette (red) ejects glutamate focally, and the evoked response is recorded at the soma. B. Glutamate-evoked currents become restricted to the site of synaptic contacts (green) after the dendrites become innervated. White dots show the positions of glutamate application; the relative distance of the yellow dot indicates the evoked current magnitude for each position. Representative glutamate-evoked currents are shown (cyan). When neurons are cultured in the presence of TTX to block all action potentials, the synaptic localization of glutamate receptors occurs nonetheless.
Organization of the postsynaptic scaffold at Glu synapses
Fig. 8.26 Postsynaptic scaffold of glutamatergic synapses. Transynaptic signaling from presynaptic neurexin (NRXN) to postsynaptic neuroligin (NLGN) can influence receptor aggregation via NLGN interaction with PSD-95. NMDA receptors (NMDAR) can interact directly with a PDZ domain on PSD-95.
AMPA receptors (AMPAR) first become anchored via an interaction with SAP-102, and transmembrane
AMPAR regulatory proteins (TARPs). Ephrin-eph receptor signaling also facilitates NMDAR and AMPAR clustering. PSD-95 and SAP-102 interact with additional scaffold proteins such as GKAP and Shank. Actinbinding proteins connect the scaffold to actin filaments.
Gephyrin clusters Gly receptors at inhibitory synapses
Fig. 8.27 Scaffold proteins support receptor clustering at inhibitory synapses. A. Gephyrin is required for glycine receptor (GlyR) clustering via a direct interaction with the β subunit. When spinal neurons are grown in culture, the peripheral membrane protein, gephyrin, colocalizes with glycine receptor clusters (top). However when translation of the gephyrin protein is blocked with an antisense oligonucleotide (bottom), the glycine receptors largely remain in the cytoplasm and clusters do not form. B.
Postsynaptic scaffold of GABAergic synapses. Transynaptic signaling from presynaptic neurexin (NRXN) to postsynaptic neuroligin-2 (NLGN2) can influence GABA receptor (GABAR) aggregation via NLGN interaction with collybistin and gephyrin.
GABARs that contain an α2 subunit can interact directly with gephyrin. GABARs that contain a γ2 subunit can cluster with gephyrin via an unidentified (?) binding protein. GABARs containing an α5 subunit are localized to extrasynaptic compartments through direct interaction with radixin. Gephyrin interacts directly with microtubules, and actin-binding proteins connect the gephyrin scaffold to actin filaments.
New AChRs are inserted into synaptic membrane after innervation red= rhoda BTX at time zero yellow= fluorescein-
AChR mAb at 8 hr
Fig. 8.28 Insertion of new ACh receptors occurs within hours of innervation. A. Cultures of muscle cells were prelabeled with rhodamine-conjugated bungarotoxin (red). B. In one set of cultures, motor neurons were added (left), while a second set of cultures remained without neurons (right). C. After eight hours, both cultures were labeled with a fluorescein-conjuctated antibody against AChRs (yellow). The cultures with motor neurons contained many AChRs that were labeled with only antibody (yellow), indicating that they had been newly inserted after the addition of motor neurons. The muscle cell cultures had AChRs that were primarily labeled by both Rhod-Btx (red) and Fluor-MAb (yellow).
AChRs accumulate outside of the synaptic membrane without neuronal activity high Ca 2+ : CaMKII myogenin-P i
no transcription of AChR in non-synaptic muscle cell nuclei
TTX: blocks synaptic activity at NMJ many extrasynaptic AChRs
Aggregation (agrin) vs. dispersion (ACh) signals at the NMJ
*increased synaptic activity causes decreased levels of AChR at the synapse
Activity-dependent decreases in AMPAR levels at Glu synapses
Arc protein is increased at active Glu synapses to promote
AMPAR degradation
Fig. 8.33 Glutamatergic synaptic activity leads to increased translation of Arc, a cytoskeleton-associated protein (red, left). The Arc increases the endocytotic removal of AMPARs from the synaptic membrane (middle).
During this time, the expression of E3 ubiquitin protein ligase (Ube3A) gradually rises (blue, middle). Ube3A attaches a polyubiquitin chain to Arc, marking it for degradation by a proteosome, and terminating AMPAR internalization.