Thin layer chromatography
advertisement
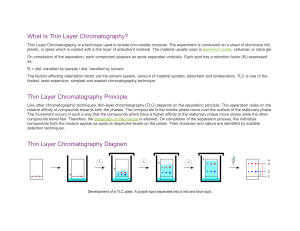
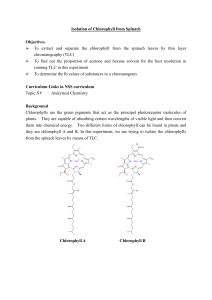
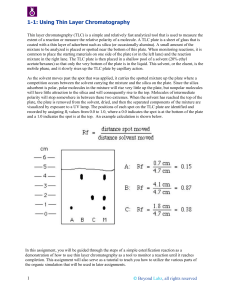
Thin layer chromatography (TLC) is a Chromatography technique used to separate mixtures. Thin layer chromatography is performed on a sheet of glass, plastic, or aluminum foil, which is coated with a thin layer of adsorbent material, usually silica gel, aluminum oxide, or cellulose (blotter paper. This layer of adsorbent is known as the stationary phase. After the sample has been applied on the plate, a solvent or solvent mixture (known as the mobile phase) is drawn up the plate via capillary action. Because different analytes ascend the TLC plate at different rates, separation is achieved. Thin layer chromatography can be used to: • Monitor the progress of a reaction • Identify compounds present in a given substance • Determine the purity of a substance Thin Layer Chromatography - t.l.c. • Series of spots forms • Compare samples in mixture with known substances. • Measure Rf values. • Coloured compounds & colourless compounds. Separation and identification. Examples on TLC separations 1. Separation of black ink on a TLC 2. The chromatography of an extract of green leaves (for example spinach) 3. The Chromatogram of 10 essential oils colored with vanillin reagent. Preparative TLC • TLC can also be used on a small semi-preparative scale to separate mixtures of up to a few hundred milligrams. The mixture is not "spotted" on the TLC plate as dots, but rather is applied to the plate as a thin even layer horizontally to and just above the solvent level. When developed with solvent the compounds separate in horizontal bands