Chem 163 Ready for class? Transition elements

Chem 163 Ready for class? Transition elements
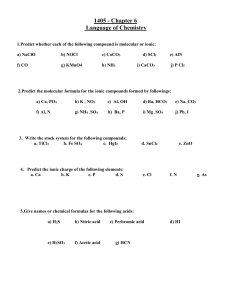
1.
The most abundant element in the earth’s atmosphere is ______________ and The most abundant metal ion in seawater is _________.
2.
What is the number of unpaired electrons in an octahedral, high-spin Mn(II) complex?
3.
O
3 and O
2
are different form of oxygen. They are called __________________________.
4.
In the diamond structure, the hybrid orbital set used by the carbon atoms to form σ-bonds is
________ and In the graphite structure, the hybrid orbital set used by the carbon atoms to form σbonds is ___________.
5.
Determine the number of unpaired electrons in an octahedral, low-spin Cr(II) complex.
6.
The ground state electron configuration of Zn 2+ is ___________________________
7. List the following in order of decreasing stability. O, O
3,
O
2
. _____________________
8.
The charge on the metal ion in the coordination complex ion, [Co(CO
3
)
3
] 3, is __________.
9.
The oxidation numbers of cobalt in the compound [Co(NH number is _________.
3
)
5
Cl]Cl
2 is ______ and the coordination
10.
The coordination number of platinum in the complex ion, [Pt(C
2
O
4
)
2
] 2, is _____.
11.
Chelating ligands are __________________________________________ (definition) and the example of a chelating legand is ____________.
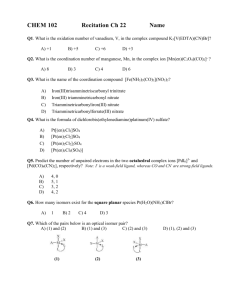
12. The name for the transition metal complex [Fe(CN)
6
] 3-
a. hexacyano ferrate(III) b. hexacyanoiron(II) c. iron(III) hexacyanide e. ferric cyanide d. ferrous cyanide
13. A coordination compound formed from cobalt(III) sulfate and ammonia contains six ammonia molecules as ligands. The formula of the compound is a. Co
2
(SO
4
)
3
∙6NH
3 b. [Co(NH
3
)
6
]
2
(SO
4
)
3 c. Co
3
SO
4
(NH
3
)
6 d. Co
2
(SO
4
)
3
(NH
4
)
6 e. Co
2
[(NH
3
)
6
](SO
4
)
3
14. Consider the following data, taken at 25 ° C
Au
2
O
3
(s) Au(s) O
2
(g)
H o f
-144.8 kJ mol -1 0.00 kJ mol -1 0.00 kJ mol -1
S o +0.1255 kJ mol -1 K 0.04741 kJ mol -1 K -1 0.205 kJ mol -1 K -
The decomposition reaction for Au
2
O
3
(s) is: 2 Au
2
O
3
(s)
→
4 Au(s) + 3 O
2
(g). Using the data given here, what is the equilibrium temperature for the reaction?
15. (a) Sketch the 5 d orbitals, and label them.
(b) Explain why d orbitals split (no longer all degenerate) in complex ions.
16.
(a) Is [Fe(H
2
O)
6
] +2 paramagnetic or diamagnetic?
(b) Is [Fe(CN)
6
] -4 paramagnetic or diamagnetic?
(c) List at least two things that are common in both of theses diagrams.
(d) One thing that is different is how 6 electrons fill the orbitals in different order. Give two reasons why.