Quiz 1 (10 pts) Chemistry 161 – Dr. Price Name __________________________
advertisement
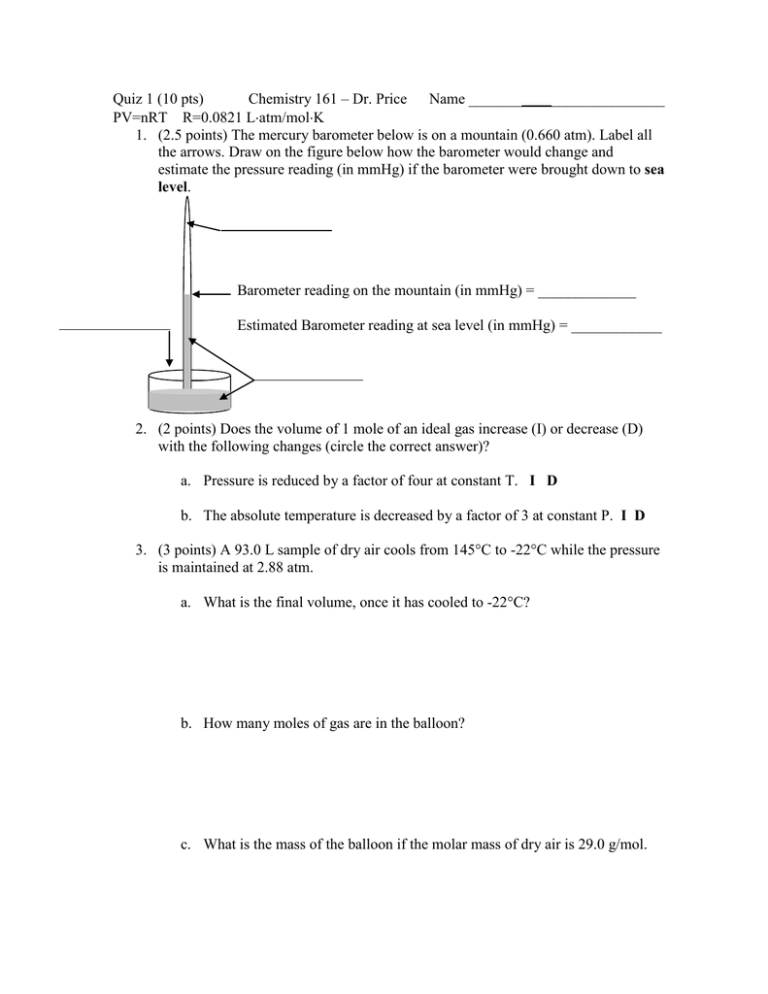
Quiz 1 (10 pts) Chemistry 161 – Dr. Price Name __________________________ PV=nRT R=0.0821 Latm/molK 1. (2.5 points) The mercury barometer below is on a mountain (0.660 atm). Label all the arrows. Draw on the figure below how the barometer would change and estimate the pressure reading (in mmHg) if the barometer were brought down to sea level. Barometer reading on the mountain (in mmHg) = _____________ Estimated Barometer reading at sea level (in mmHg) = ____________ 2. (2 points) Does the volume of 1 mole of an ideal gas increase (I) or decrease (D) with the following changes (circle the correct answer)? a. Pressure is reduced by a factor of four at constant T. I D b. The absolute temperature is decreased by a factor of 3 at constant P. I D 3. (3 points) A 93.0 L sample of dry air cools from 145°C to -22°C while the pressure is maintained at 2.88 atm. a. What is the final volume, once it has cooled to -22°C? b. How many moles of gas are in the balloon? c. What is the mass of the balloon if the molar mass of dry air is 29.0 g/mol. 4. (2.5 points) For the sample gas mixture pictured below, , , and shapes represent individual gas molecules. Answer the following. i. What is the mole fraction of ?__________ ii. Which gas has the highest partial pressure?_______ iii. Which gas has the lowest partial pressure?______ iv. If the total pressure is 0.75 atm, what is the partial pressure for ?____________ v. Would removing all the ‘s change or not change the partial pressures of the remaining gases? Explain.