Chem 121 In-class Assignment #3 Names: _______________________________________________________________________
advertisement
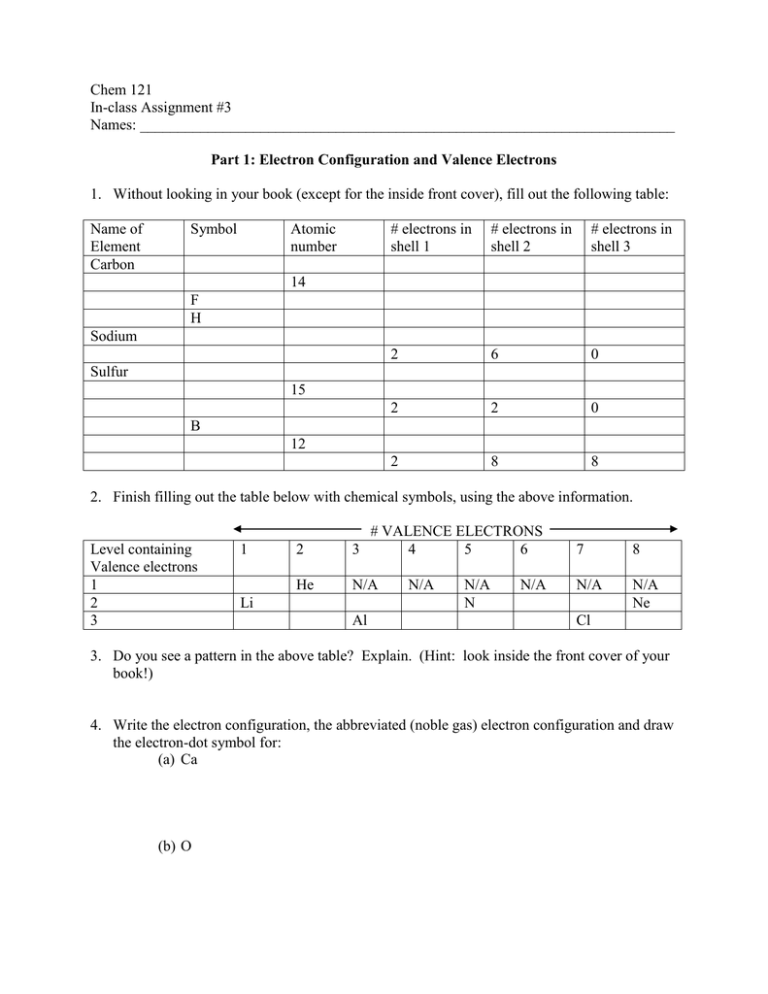
Chem 121 In-class Assignment #3 Names: _______________________________________________________________________ Part 1: Electron Configuration and Valence Electrons 1. Without looking in your book (except for the inside front cover), fill out the following table: Name of Element Carbon Symbol Atomic number # electrons in shell 1 # electrons in shell 2 # electrons in shell 3 2 6 0 2 2 0 2 8 8 14 F H Sodium Sulfur 15 B 12 2. Finish filling out the table below with chemical symbols, using the above information. Level containing Valence electrons 1 2 3 1 2 # VALENCE ELECTRONS 3 4 5 6 7 8 He N/A N/A N/A Ne Li Al N/A N/A N N/A Cl 3. Do you see a pattern in the above table? Explain. (Hint: look inside the front cover of your book!) 4. Write the electron configuration, the abbreviated (noble gas) electron configuration and draw the electron-dot symbol for: (a) Ca (b) O Part 2: Ions and Ionic Compounds 1. Fill out the table for the following elements that form anions: Name of Element Symbol Phosphorous Oxygen Fluorine Chlorine P O F Cl # of valence electrons # of electrons needed to GAIN to have an octet Symbol of ion 2. Fill out the table for the following elements that form cations: Name of Element Symbol Calcium Aluminum Beryllium Iron Ca Al Be Fe # of valence electrons # of electrons needed to LOSE to have an octet Symbol of ion 3. Give the name AND the formula of the ionic compound that will form from the following ions. Identify the anion and the cation. (a) The ion formed when Mn loses 2 electrons, and the ion formed from iodine (b) The ion formed from Ca and the ion formed from chlorine (c) Nitrate, NO3-, and the ion formed when iron loses 2 electrons 4. Is it possible for Na and Al to form an ionic compound together? Why or why not?

