0-D, 1-D, 2-D Structures NANO 101 Introduction to Nanotechnology
advertisement

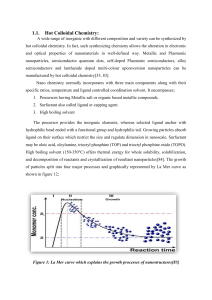
0-D, 1-D, 2-D Structures (not a chapter in our book!) NANO 101 Introduction to Nanotechnology 1 Overview Bottom Up Top Down Chemistry! Milling Large size distribution • No control of shape • Impurities • Crystal Growth • 0-D particles • 1-D particles • 2-D films Lithography 2 Top-Down Approaches • Milling – Broad size distribution (tens to hundreds of nm) – Varied shape and geometry – Impurities and defects from milling • Lithography – Also includes bottom up method https://sites.northwestern.edu/vanduyne/files/2012/10/2001_Haynes_4.pdf 3 Particle Requirements • Uniform size • Uniform morphology • Uniform chemical composition and crystal structure • Monodispersed 4 Homogeneous Nucleation/ Supersaturated solution kT C G ln Co G = Gibbs free energy K = Boltzmann constant Co = equilibrium concentration T = temperature Ω = atomic volume Two competing forces • Surface energy • Volume energy N&N Fig. 3.2 5 Nucleation and Growth Rates N&N Fig. 3.4 6 Hot Injection • A way to separate nucleation and growth: – One ionic precursor is heated to ~ 300 C – Other precursor is a room temp and injected – Rapid nucleation occurs followed by temperature drop and growth phase 7 Hot Injection 8 Growth of Nanoparticles 9 Chem. Rev., 2014, 114 (15), pp 7610–7630 Making Nanoparticles D i f f u s i o n Growth 1. Nucleation 2. Diffusion from bulk to surface 3. Adsorption to surface 4. Irreversible incorporation onto surface If the slowest step is diffusion uniform particles If the slowest step is layer by layer growth nonuniform particles 10 Favoring Diffusion-limited Growth • Low concentrations – Large diffusion distance • High solution viscosity • Introduce diffusion barrier • Change rate of chemical reactions – Reactants used – Catalysts 11 Other Strategies: • Heat up method – in situ formation of reactive precursors • Slow addition of precursors – for RT growth 12 0-D Nanostructures: Surface Area and Energy Surface energy increases with surface area • • C. Nutzenadel et al., Eur. Phys. J. D. 8, 245 (2000). Large surface energy = instability Driven to grow to reduce surface energy 13 Electrostatic Stabilization Establish Surface Charge Density Adsorption of ions/charged species + - + - + - + + + - - + + + + + + + + - + + + + + - + - + + + + • 14 Steric Stabilization “Capping” Anchored • Irreversible binding Adsorbed • Random, weak 15 What is on the surface? • Current area of research: Probing the surface of platinum nanoparticles with 13CO by solid-state NMR and IR spectroscopies Nanoscale, 2014,6, 539-546 16 Example: Colloidal Gold • Comprehensive study on synthesis and properties of colloidal gold published by Faraday (1857) • Classic method – Precursor: dilute chlorauric acid (HAuCl4) – Reducing agent: sodium citrate (NaC6H5O7) – Reaction temperature: 100 °C – Product: stable, uniform, ~20 nm particles 17 Colloidal Gold Particle Size 18 N&N Fig. 3.9 Colloidal Gold Particle Size 19 N&N Fig. 3.9 Synthesis of Metallic Nanoparticles • Reduction of metal complexes in dilute solutions • Precursors – Elemental metals, inorganic salts, metal complexes • Reduction agents • Stabilizers – PVA – Sodium polyacrylate 20 Other Methods • Brust Synthesis •Reverse Micelle 21 Influence of “Capping” • • • • • Addition of polymer stabilizer Used on surface to prevent agglomeration Affects growth by limiting growth site May interact with solute, catalyst, solvent Can affect morphology N&N Fig. 3.13 22 Growth of Pt Nanoparticles • Found that ligands can terminate growth instead of change growth rate. 23 Influence of Temperature N&N Fig. 3.14 24 Influence of Concentration J. Phys. Chem. B, Vol. 108, No. 40, 2004 25 Influence of Time Rhodium nanocrystals J. Phys. Chem. C, Vol. 111, No. 16, 2007 26 Influence of pH Initial pH of reaction can affect size SnO2 J. Phys. Chem. B, Vol. 108, No. 40, 2004 27 Formation of Nanoparticles in Solution Advantages: 1. Stabilization from agglomeration 2. Extraction of nanoparticles from solvent 3. Surface modification and application 4. Mass production 28 MBE Quantum Dots http://www.nanowerk. com/nanotechnologynews/newsid=37518.p hp http://www.mbe.ethz. ch/index.php?id=mbe • Self-assemble due to lattice mismatch 29 How are these 0D? GaAs GaAs In As E 30 Formation of Nanoparticles on Substrates • Advantages: – No ligands needed – Very stable – Ready for electronic application – Access different materials easily 31