Problem 2
advertisement
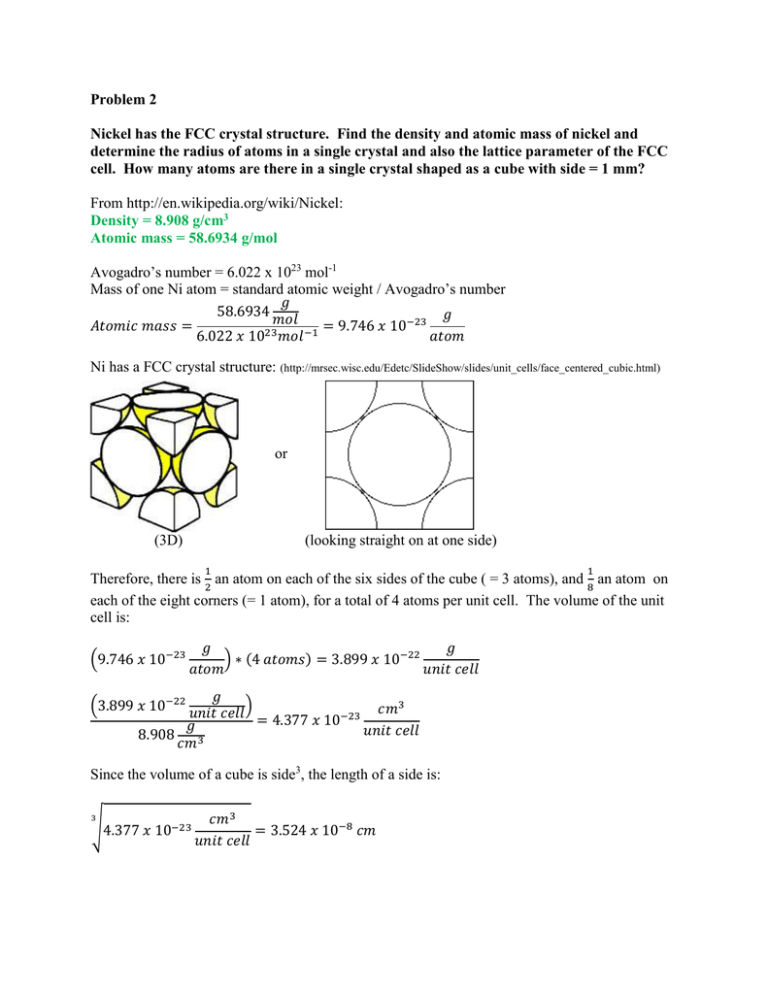
Problem 2 Nickel has the FCC crystal structure. Find the density and atomic mass of nickel and determine the radius of atoms in a single crystal and also the lattice parameter of the FCC cell. How many atoms are there in a single crystal shaped as a cube with side = 1 mm? From http://en.wikipedia.org/wiki/Nickel: Density = 8.908 g/cm3 Atomic mass = 58.6934 g/mol Avogadro’s number = 6.022 x 1023 mol-1 Mass of one Ni atom = standard atomic weight / Avogadro’s number 𝑔 58.6934 𝑔 𝑚𝑜𝑙 −23 𝐴𝑡𝑜𝑚𝑖𝑐 𝑚𝑎𝑠𝑠 = = 9.746 𝑥 10 6.022 𝑥 1023 𝑚𝑜𝑙 −1 𝑎𝑡𝑜𝑚 Ni has a FCC crystal structure: (http://mrsec.wisc.edu/Edetc/SlideShow/slides/unit_cells/face_centered_cubic.html) or (3D) (looking straight on at one side) 1 1 Therefore, there is 2 an atom on each of the six sides of the cube ( = 3 atoms), and 8 an atom on each of the eight corners (= 1 atom), for a total of 4 atoms per unit cell. The volume of the unit cell is: (9.746 𝑥 10−23 𝑔 𝑔 ) ∗ (4 𝑎𝑡𝑜𝑚𝑠) = 3.899 𝑥 10−22 𝑎𝑡𝑜𝑚 𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙 𝑔 3 (3.899 𝑥 10−22 ) 𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙 = 4.377 𝑥 10−23 𝑐𝑚 𝑔 𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙 8.908 𝑐𝑚3 Since the volume of a cube is side3, the length of a side is: 3 √4.377 𝑥 10−23 𝑐𝑚3 = 3.524 𝑥 10−8 𝑐𝑚 𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙 Drawing a diagonal line through the cube, we see that there are 4 radii of Ni atom along the hypotenuse of a triangle, as shown below: Using the Pythagorean theorem, the hypotenuse ( = 4 radii) is: 𝑠𝑖𝑑𝑒 2 + 𝑠𝑖𝑑𝑒 2 = ℎ𝑦𝑝𝑜𝑡𝑒𝑛𝑢𝑠𝑒 2 2𝑠𝑖𝑑𝑒 2 = ℎ𝑦𝑝𝑜𝑡𝑒𝑛𝑢𝑠𝑒 2 2(3.524 𝑥 10−8 𝑐𝑚)2 = ℎ𝑦𝑝𝑜𝑡𝑒𝑛𝑢𝑠𝑒 2 2.484 𝑥 10−15 𝑐𝑚 = ℎ𝑦𝑝𝑜𝑡𝑒𝑛𝑢𝑠𝑒 2 4.984 𝑥 10−8 𝑐𝑚 = ℎ𝑦𝑝𝑜𝑡𝑒𝑛𝑢𝑠𝑒 = 4 𝑟𝑎𝑑𝑖𝑖 Thus, 1 𝒓𝒂𝒅𝒊𝒖𝒔 𝒐𝒇 𝑵𝒊 𝒂𝒕𝒐𝒎 = 𝟒.𝟗𝟖𝟒 𝒙 𝟏𝟎−𝟖 𝒄𝒎 𝟒 = 𝟏. 𝟐𝟒𝟔 𝒙 𝟏𝟎−𝟖 𝒄𝒎 The lattice parameters are the unit cell side lengths and angles between them. For a FCC crystal structure, all sides are the same length, and all angles between the sides are the same. (http://www.doitpoms.ac.uk/tlplib/crystallography3/parameters.php) 𝒂 = 𝒃 = 𝒄 = 𝟑. 𝟓𝟐𝟒 𝒙 𝟏𝟎−𝟖 𝒄𝒎 𝜶 = 𝜷 = 𝜸 = 𝟗𝟎° 𝑐𝑚3 If there are 4 atoms in a cube with volume = 4.377 𝑥 10−23 𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙, then for a cube with side of 𝑐𝑚3 1 mm, or volume = 1 𝑚𝑚3 = 0.001 𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙, there will be: 4 𝑎𝑡𝑜𝑚𝑠 4.377 𝑥 10−23 𝑐𝑚3 𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙 = 𝑋 𝑎𝑡𝑜𝑚𝑠 𝑐𝑚3 0.001 𝑢𝑛𝑖𝑡 𝑐𝑒𝑙𝑙 𝑋 = 𝒂𝒕𝒐𝒎𝒔 𝒊𝒏 𝒂 𝒄𝒖𝒃𝒆 𝒘𝒊𝒕𝒉 𝒂 𝒔𝒊𝒅𝒆 𝒐𝒇 𝟏𝒎𝒎 = 𝟗. 𝟏𝟑𝟗 𝒙 𝟏𝟎𝟏𝟗 𝒂𝒕𝒐𝒎𝒔





