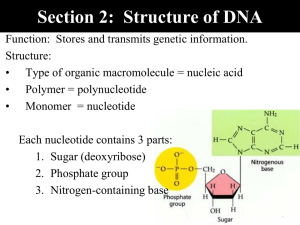
Name:____________________________ Fall Semester, 2004 Exam I I. Multiple choice (80 points)
advertisement

Name:____________________________ Fall Semester, 2004 Exam I I. Multiple choice (80 points) 1. The net yield of ATP from a glucose molecule being metabolized through glycolysis to pyruvate is ______ molecule(s) . a. 1 b. 2 c. 4 d. 36 e. 42 2. 3. Glycolysis occurs: a. on b. on c. in d. in e. in the the the the the plasma membrane. mitochondrial inner membrane. mitochondrial matrix. mitochrondrial intermembrane space. cytoplasm. In addition to ATP, glycolysis produces: a. GTP. b. NADH. c. lactate. d. FADH2. e. a proton gradient across the membrane 4. At what point during cell division does synapsis occur? a. prophase I of meiosis b. telophase I of meiosis c. metaphase II of meiosis d. prophase of mitosis e. anaphase I of meiosis 5. In which phase of meiosis do the homologous chromosomes undergo separation? a. metaphase I b. anaphase II c. prophase I d. metaphase II e. anaphase I During which phase of meiosis are the tetrads of homologous chromosomes aligned at the center of the dividing cell? a. prophase I b. metaphase I c. anaphase II d. anaphase I e. metaphase II 33. During which phase of meiosis do the centromeres uncouple and sister chromatids separate? a anaphase I b. prophase II c. metaphase II d. anaphase II e. metaphase I 34. Between the metaphases of meiosis I and meiosis II,: a. b. c. d. there is a full cell cycle. there is an extended G1 period. the nuclear membranes do not reform. a decrease in chromosome number has occurred. e. a single round of DNA replication is carried out ATP is synthesized by ATP synthase in the mitochondria by the process of: A. chemiosmosis. B. electron transport. C. substrate level phosphorylation. D. oxidative phosphorylation. E. redox reactions. 40. A human gamete has: A. 23 chromosomes. B. 46 chromosomes (23 pairs). C. 46 chromosomes. D. 23 autosomes. E. two sex chromosomes. The two sister chromatids of a eukaryotic chromosome are connected at the: A. centromere. B. centriole. C. chiasma. D. telomere. E. centrosome. 42. 44. 45. 46. DNA replication occurs in eukaryotic cells during: a. G1 phase. b. S phase. c. G2 phase. d. mitosis. e. G0 phase. The pairing of homologous chromosomes during meiosis is called: a. partitioning. b. synapsis. c. chiasma. d. pleiotropy. e. epistasis. Sexual life cycles produce genetic variation in offspring by: a. independent assortment of chromosomes. b. crossing over between nonsister chromatids. c. random fertilization. d. Only two of the above answers are correct e. All of the above answers are correct. Gametes are examples of: a. haploid cells. b. somatic cells. c. diploid cells. d. the products of mitotic division. e. 52. things your parents donÕt want to talk about The final acceptor for the mitochondrial electron transport chain is: a. water. b. oxygen. c. NAD. d. ATP. 54. e. ADP. The electrons from NADH, when fed through the electron transport chain of mitochondria, provide a theoretical yield of: a. 1 ATP. b. 2 ATP. c. 3 ATP. d. 4 ATP. e. 1 oxygen. 1. Snapdragons have a sincle gene (locus) that determines flower color. The allele R is for red flowers and it shows incomplete dominance over the recessive allele r for white flowers. What color flowers are produced by Rr plants? a. All red b. Mixed red and white (some flowers of each color) c. pink d. white with pink streaks e. purple 2. An allele at one locus affects several phenotypic traits (e.g. cystic fibrosis is caused by a single defective gene which causes clogged blood vessels, sticky mucus, salty sweat, liver failure, etc.). This is an example of: a. continuous variation. b. codominance. c. incomplete dominance. d. epistasis. e. pleiotropic effects. 3. A human is heterozygous at a blood group locus and expresses both genotypes (e.g. they have type AB blood). This is an example of: a. polygenic inheritance. b. codominance. c. incomplete or partial dominance. d. pleiotropy. e. complete dominance. 5. An example of a sex-linked human genetic-based disorder is: a. Duchene muscular dystrophy. b. sickle cell anemia. c. HuntingtonÕs disease. d. cystic fibrosis. e. Tay-Sachs disease. Genes located on the same chromosome are said to be: a. polygenic. b. bottlenecked. c. pleiotropic. d. linked. e. epistatic. 6. 7. A human autosomal recessive lethal genetic disease whose defective allele has been maintained at a relatively high level in certain population groups because it gives the heterozygote resistance to an infectious disease is: a. Tay-Sachs disease. b. cystic fibrosis. c. sickle-cell anemia. d. muscular dystrophy. e. HuntingtonÕs disease. 9. Thomas Morgan was a. b. c. d. e. 10. An example of a human genetic condition caused by an autosomal dominant defective gene/allele: a. KlinefelterÕs syndrome. b. Down syndrome. c. HuntingtonÕs disease. d. cystic fibrosis. e. Turner. 11. An inactivated ÒXÓ chromosome in a human female cell is seen as a/an: a. centrosome. b. Barr body. c. genetic imprint. d. nucleosome. e. centromere. An example of a human genetic disease involving a male with a Barr body is: a. Klinefelter syndrome. b. Down syndrome. c. HuntingtonÕs disease. d. Turner syndrome e. muscular dystrophy. 14. the first person to: map a specific gene/trait to a specific chromosome. observe codominance. carry out amniocentesis. observe crossing over under the microscope. define genetic variation. 15. The human chromosomes which are not either the "X" or "Y" chromosomes are collectively called _________. a. mosaics b. sex chromosomes c. karyotypes d. monosomics e. autosomes 16. A human gene is said to be sex-linked if: a. it is more common in females than males. b. it is found on the ÒYÓ chromosome. c. it is encoded by the ÒXÓ chromosome. d. it is expressed only in males. 17. The structure shown to the right is an example of a: A. purine. B. pyrimidine. C. ribose sugar. D. deoxyribose sugar. 23. During DNA replication, the "lagging strand" is synthesized as a series of small DNA fragments which are later connected into a continuous DNA molecule. These short segments are called : a. Okazaki fragments. b. replicons. c. replication forks. d. transcription units. e. Griffith units. e. E. it is recessive in males molecule not found in DNA 24. The enzyme which unwinds the DNA helix, allowing replication to occur is called: a. primase. b. DNA replicase. c. reverse transcriptase. d. helicase. 26. DNA replication in bacteria occurs by a _____________ mechanism a. semi-conservative b. conservative c. dispersive d. continuous e. none of the above The enzyme which covalently connects the short DNA fragments synthesized on the lagging strand (i.e. "ties" them together) is called: a. DNA nuclease. b. DNA polymerase. c. helicase. d. DNA ligase. e. primase. Griffith used rodents and strains of Streptococcus pneumoniae (the bacterial species he used were at that time known as Pneumococcus) to demonstrate: a. that RNA is the genetic material. b. DNA replication occurs semiconservatively. c. bacterial transformation. d. protein coats of viruses are not required for successful infection. e. German bombs can adversely affect experimental results. e. 27. 30. 31. 33. 35. 37. DNA litigase The structure of DNA molecules was first elucidated by: a. Watson and Crick. b. Avery. c. Franklin and Wilkins. d. Griffith. e. Beadle and Tatum. Which of the following is NOT true of the presently accepted model for the structure of DNA? a. It is a double helix. b. The two strands are antiparallel. c. The bases are located on the interior of the helix. d. The two strands are complementary. e. All of the above are true of this model for DNA structure. Chargaff’s studies lead him to conclude: a. that the amount of adenine (A) in the DNA from an organism always equaled the amount of thymine (T) in the DNA from the same organism. b. that DNA was a helix. c. that the lactose operon was inducible. d. that DNA was in fact the genetic material responsible for bacterial transformation. e. he should have gone to medical school and make money The X-ray crystallography pictures critical to the discovery of the structure of DNA were obtained by: a. Franklin and Wilkins. b. c. d. e. Watson and Crick. Avery and Griffith. Beadle and Tatum. Messelson and Stahl. 38. The individual nucleotides which make up a single strand of DNA are connected to the nucleotides above and below them (or to the 5Õ and 3Õ sides if you prefer--NOT CONNECTING TWO STRANDS OF THE DUPLEX to each other) by: a. peptide bonds. b. hydrogen bonds. c. phosphodiester bonds. d. reversible bonding. e. faith. 39. In a DNA duplex, the base A is always paired with: a. guanine. b. cytosine. c. thymine. d. uracil. e. deoxyribose. 40. The base pairs between two strands of a DNA molecule are held together by: a. phosphodiester bonds. b. ionic bonds involving the phosphate groups. c. polar covalent bonds. d. hydrogen bonds. e. a combination of all of the above. 43. The location on a chromosome where DNA replication begins is called a/an: a. origin (ori). b. promoter. c. centromere. d. telomere. e. initiator. 45. A population of flowers has a range of colors from white to pink to dark red. Deer eat the lighter colored plants to the point where their numbers are reduced in the population, giving the population a darker average color. This would be an example of: a. stabilizing selection. b. directional selection. c. diversifying selection. d. random selection. e. frequency-dependent selection. 49. A human with the XXY karotype is: a. phenotypically a male and no Barr body. b. phenotypically female. c. phenotypically male but with a Barr body. d. phenotypically female with no Barr body. e. frequency-dependent selection. 50. An example of a phenotypic female human with no Barr body would be someone with: a. an XXX karyotype. b. Down Syndrome. c. Kleinfelter Disease. d. e. 51. 55. Huntington Disease. Turner Syndrome. A person with a karyotype of trisomy 21 will suffer from: a. Tay-Sachs. b. Down Syndrome. c. Kleinfelter Disease. d. Huntington Disease. e. Turner Syndrome. A trait shows continuous variation within a population (e.g. humans). This suggests that the trait exhibits: a. a polygenic pattern of inheritance. b. pleiotrophy. c. incomplete dominance. d. codominance. height in e. linkage disequilibrium 1. Which of these terms refers to the process of maintaining relatively constant internal conditions in a living organism? a. Order. b. Sensitivity c. Development d. Homeostasis e. Regulation 2. The cation form of an element is compared to the uncharged form of the same element. Which of the following statements is TRUE? a. The cation has less protons. b. The cation has a different half-life c. The cation has more neutrons. d. The cation has less electrons. e. The cation has more electrons. 3. Mitochondria are believed to have evolved from: a. the golgi apparatus. b. the endoplasmic reticulum. c. invaginations of the plasma membrane d. endosymbiont bacteria. e. early photosynthetic organisms. 4. A solution with neutral pH would have a pH value of: a. 0.0 b. 1.0 c. 7.0 d. 10.0 e. 14.0 5. A substance which acts to maintain the pH of a solution at its present value, counteracting the effects of adding acid or base, is called a/an: a. b. c. d. e. anion. cation. buffer. salt. hydrophile. 6. Two atoms share 2 electrons. This is an example of: a. a single bond. b. a double bond. c. a triple bond. d. a hydrogen bond. e. None of the above is a correct answer. 7. Which of the following will increase the rate of a chemical reaction? a. Higher temperature b. Higher reactant concentration c. A catalyst d. All of the above speeds up a chemical reaction. e. None of the above speeds up a chemical reaction. 8. Water is a liquid at room temperature (and not a gas) because: a. it can form ionic bonds between ionized molecules. b. it can form hydrogen bonds between individual molecules. c. it can dissociate to form hydrogen and hydroxide ions. d. it has a relatively high molecular weight compared to gases. e. it forms a strong hydration shell. 9. A crystal of sodium chloride is held together by: a. hydrogen bonds. b. ionic bonds. c. covalent bonds. d. hydration shells of the individual molecule. e. a chemical reaction between the sodium and chloride ions. 10. Which of the above properties of water was/is not important to the development of living systems? a. Cohesion—water molecules “stick together” b. High specific heat--water requires much energy to raise its temperature c. High heat of vaporization--much energy absorbed when water evaporates d. Low density of ice—it floats e. All of the above are properties of water that are important to the development and maintenance of life on this planet. 11. The basic monomeric repeat unit which is used to assemble nucleic acids is the: a. amino acid b. sugar c. lipid d. nucleotide e. phosphate 12. An example of a structural polymer made entirely of glucose repeat units is/are: a. The cell membrane. b. Microtubules. c. The nuclear membrane. d. A ribosome. e. None of the above answers is/are correct. 13. The type of reaction commonly used to disconnect (break apart) the monomeric units of macromolecules is best classified as a/an: a. exergonic reaction. b. hydrolysis reaction. c. disulfide reaction. d. hydrogen bonding. e. dehydration reaction. 14. The actual sequence of amino acids in a polypeptide/protein is called its: a. primary structure. b. secondary structure. c. tertiary structure. d. quaternary structure. e. None of the above are correct answers. 15. An example of a type of chemical bond which involves a full separation of charge (transfer of electrons) is a/an: a. hydrogen bond. b. ionic bond. c. covalent bond. d. hydrophobic bond. e. electron bond. 16. A storage fat molecule is made up of: a. two fatty acids, glycerol, phosphate and another alcohol type unit. b. three fatty acids and glycerol. c. two fatty acids and glycerol. d. two fatty acids, glycerol and a phosphate group. e. three fatty acids, glycerol, phosphate and an alcohol type unit. 17. The transport of glucose from the plasma into a human red blood cell is accomplished by: a. active transport. b. cotransport. c. diffusion. d. facilitated diffusion. e. pinocytosis. 18. The four most common elements in living systems are: a. carbon, hydrogen, oxygen and phosphorous. b. carbon, hydrogen, nitrogen and oxygen. c. carbon, oxygen, nitrogen and phosphorous. d. carbon, oxygen, nitrogen and iron. e. carbon, oxygen, nitrogen and calcium. 19. The beta-sheet and alpha-helix of a folded protein/polypeptide would be examples of its: a. primary structure. b. secondary structure. c. tertiary structure. d. quaternary structure. e. All of the above are correct answers. 20. An example of a storage carbohydrate common in plants is: a. cellulose. b. glycogen. c. starch. d. chitin. e. choline. 21. The atomic number of an element refers to the: a. number of protons in the nucleus. b. number of protons plus neutrons in the nucleus. c. number of neutrons in the nucleus. d. number of electrons in the outer shell/orbital. e. number of covalent bonds it routinely makes with other atoms. 22. (CH2O)n is the common formula for: a. amino acids. b. sugars. c. fatty acids. d. hydrophylic molecules. e. hydrophobic molecules. 23. The charged particle with a mass of approximately 1 in the nuclei of atoms is called a/an: a. neutron b. proton c. nucleus d. electron e. neutrino 24. Biological membranes are composed of [choose the best answer]: a. fats. b. c. d. e. a peptidoglycan layer. a phospholipid bilayer. Carbohydrate fibers related to cellulose. All of the above are correct answers. 25. Ribose is an example of a/an: a. purine. b. pyrimidine. c. sugar. d. aldehyde. e. fat. 26. Which of the following is a nucleotide? a. ATP b. A fat c. A sugar d. A phospholipid e. Cholesterol 27. The monomer repeat units of a protein are connected to each other by what type of bonds? a. Peptide bonds b. Phosphodiester bonds c. Ester bonds d. Hydrogen bonds e. Ionic bonds 28. What is the defining characteristic of an acid? a. It donates hydrogen ions. b. It has an excess of hydroxide ions c. It accepts hydrogen ions. d. it has a pH greater than 7. e. It will donate or accept hydrogen ions depending on the pH. 29. If two atoms share a pair of electrons, they are held together by a(n) a. polar bond b. hydrogen bond c. neutral bond d. covalent bond e. ionic bond 30. Which of the following is a CORRECT statement about the scientific method? a. it distinguishes between good and bad b. it can only be done by someone with a Ph.D. or advanced training c. it organizes evidence and helps us predict what will happen in our environment d. it requires expensive laboratory equipment e. its methods are substantially different from the way people normally process information 31. Based upon your knowledge of atomic structure, an atom with 14 protons in its nucleus a. would be very stable and unreactive b. would be very positively charged c. tend to form covalent bonds d. would probably form ionic bonds e. none of the above 32. Each amino acid differs from others in the a. location of the carbon atoms b. nature of the R group c. number of carboxyl groups d. all of the above e. size of the amino group 33. The bonds between two carbon atoms or carbon and hydrogen represents a. shared electrons b. energy sinkholes c. both a and d d. slurpy factoids e. ionic bonds 34. Which of the following lists the four elements that make up 97% of all living tissue? a. hydrogen, carbon, nitrogen, oxygen b. carbon, hydrogen, nitrogen, sulfur c. oxygen, carbon, helium, nitrogen d. nitrogen, carbon, helium, sulfur e. oxygen, nitrogen, carbon, sulfur 35. Pure water has a pH of 7 because it contains a. equal numbers of hydrogen and hydroxide ions b. none of the above c. equal numbers of hydrogen and oxygen atoms d. a, b, and c are correct e. a variety of buffers 36. You drink a glass of lemonade, but your body's pH does not change. This is an example of how organisms: a. maintain homeostasis. b. are immune to acid c. maintain organization d. are what they eat. e. adapt to their environment. _______________________________________________________________________ Match the following names to the proper functional group a. -OH d. -C=O b. -SH e. -NH3 c. -PO4 37. A sulfhydryl group 38. An amino group 39. A carbonyl group 40. A hydroxyl group _______________________________________________________________________ II. (10 points) Fill in the blanks with the correct word or statement. The value of each question is in the parentheses following the number. 1. (1) The linear arrangement of amino acids in the polypeptide chain is referred to as the __________________ structure of the protein. 2. (1) Cholesterol, testosterone, and estrogen are all examples of _______________________________. 3. (2) List two of the attributes something must have to be considered truly alive: __________________________________ and __________________________________________________. 4. (1) A compound that maintains a solution at a constant pH by accepting or releasing H+ in response to small changes in H+ concentrations is known as a __________________________. 5. (1) pH is the measure of the concentration of ________________________ present in a solution. 7. (1) Name one function of carbohydrates _______________________________________________ 8. (1) Water is attracted to any substance which is _______________________________________ 9. (1) Name one function of nucleic acids ________________________________________ 10. (1) The 3 parts of a nucleotide are a nitrogen, a phosphate group and a __________________ III. (5 points) Matching. Place the letter of the correct answer into the blank to the left of the term. _____1. glucose _____2. steroid _____3. hydroxyl group (A) (B) _____4. phospholipid _____5. amino acid (C) (D) (E) (F) IV. (5 pts). Label the parts of the microscope -OH (G) -COOH (H) -NH2