The A.M.I. School Class: IX Atomic Structure (Practice)
advertisement
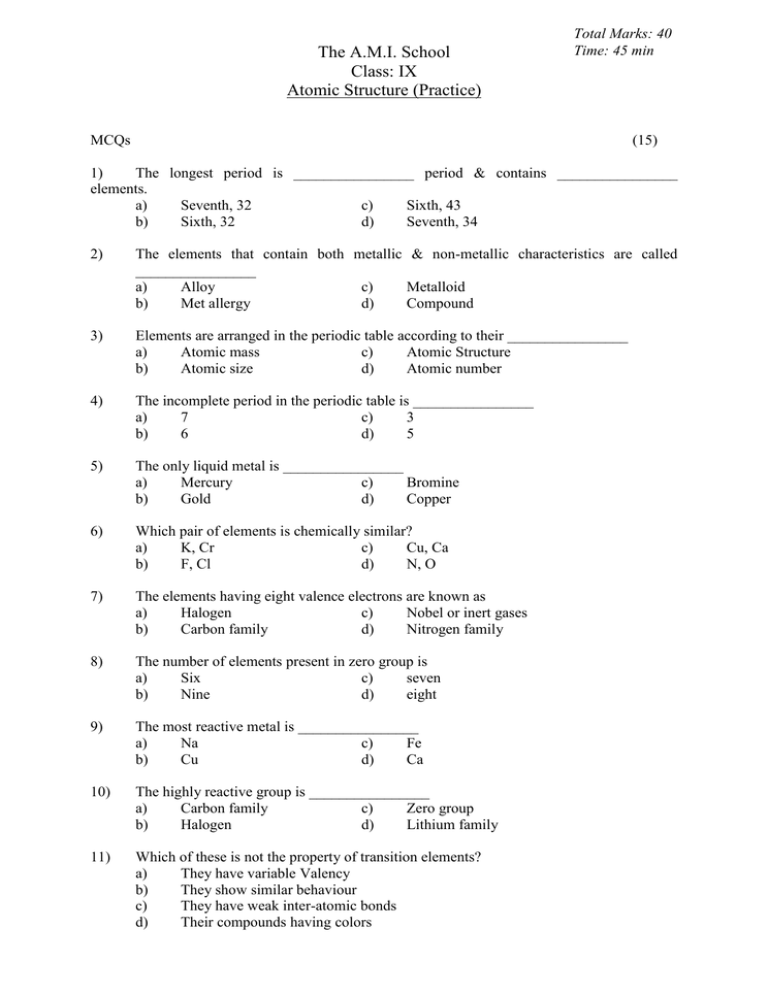
The A.M.I. School Class: IX Atomic Structure (Practice) Total Marks: 40 Time: 45 min MCQs (15) 1) The longest period is ________________ period & contains ________________ elements. a) Seventh, 32 c) Sixth, 43 b) Sixth, 32 d) Seventh, 34 2) The elements that contain both metallic & non-metallic characteristics are called ________________ a) Alloy c) Metalloid b) Met allergy d) Compound 3) Elements are arranged in the periodic table according to their ________________ a) Atomic mass c) Atomic Structure b) Atomic size d) Atomic number 4) The incomplete period in the periodic table is ________________ a) 7 c) 3 b) 6 d) 5 5) The only liquid metal is ________________ a) Mercury c) Bromine b) Gold d) Copper 6) Which pair of elements is chemically similar? a) K, Cr c) Cu, Ca b) F, Cl d) N, O 7) The elements having eight valence electrons are known as a) Halogen c) Nobel or inert gases b) Carbon family d) Nitrogen family 8) The number of elements present in zero group is a) Six c) seven b) Nine d) eight 9) The most reactive metal is ________________ a) Na c) Fe b) Cu d) Ca 10) The highly reactive group is ________________ a) Carbon family c) Zero group b) Halogen d) Lithium family 11) Which of these is not the property of transition elements? a) They have variable Valency b) They show similar behaviour c) They have weak inter-atomic bonds d) Their compounds having colors 12) An atom is considered as neutral because a) Positive & negative charges cancelled each other b) Nucleus of an atom carries positive charge c) Neutron has no charge d) Electrons carry negative charge 13) Which one of the appropriate characteristic of Alkali metals. a) Outer most shell having 2 electrons b) Highly reactive c) They show more than one form of Allotropy d) They all are non reactive 14) Change of an electron expressed in a) Gm c) b) Coulombs d) joules kilo-watt 15) The scientist was the first chemist to put elements in a periodic table. a) Robert Brown c) Mosley b) Rutherford d) Mendeelve Q.1) Define the elements & how many element on the earth have been discovered? (01) ________________________________________________________________________ ________________________________________________________________________ Q.2) Difference between metals & Non-metals (04) Metal . _______________________________ Non-metal _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ Q.3) In what ways Isotopes of an element differ from each other. (03) Isotopes have (Different) Q.4) Calculate the number of neutrons in the following element or Isotopes. Elements / Isotopes 15 7 (04) N=A-Z N 18 8 O 3 1 D 35 17 Cl Q.5) Give three characteristics of Halogen (03) ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ ________________________________________________________________________ Q.6) Label the structure of the periodic table as groups, periods & mention the names of groups also: (10)