CHEMICAL EQUATIONS TEST REVIEW H₂O C₆H₁₂O₆
advertisement
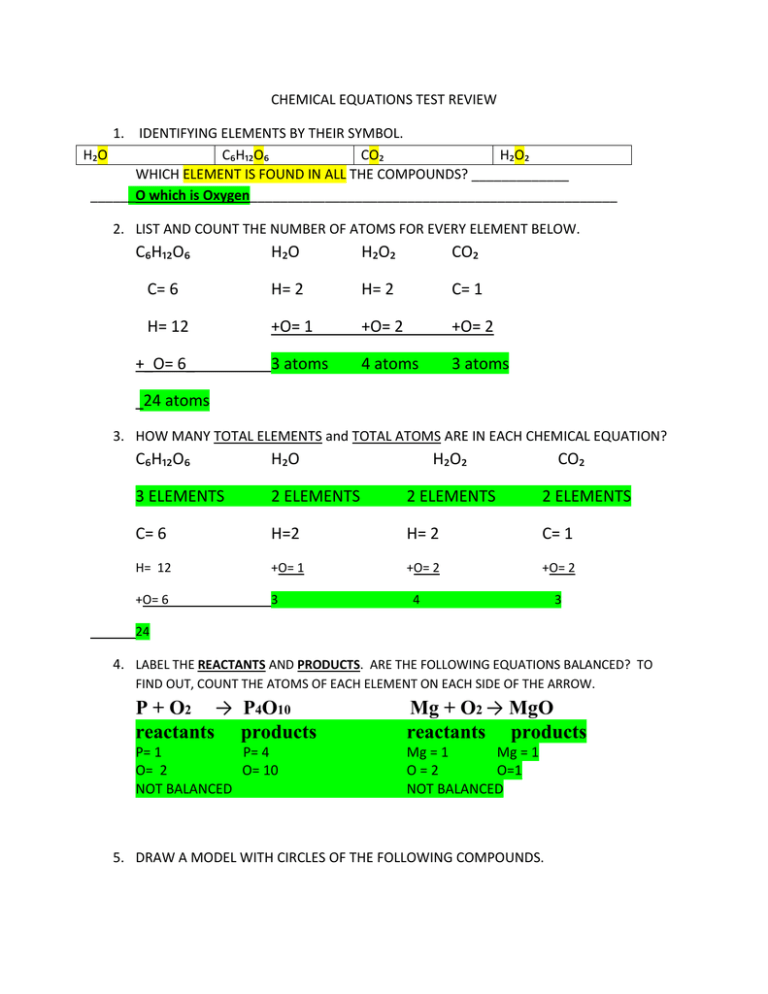
CHEMICAL EQUATIONS TEST REVIEW 1. IDENTIFYING ELEMENTS BY THEIR SYMBOL. H₂O C₆H₁₂O₆ CO₂ H₂O₂ WHICH ELEMENT IS FOUND IN ALL THE COMPOUNDS? _____________ ______O which is Oxygen_________________________________________________ 2. LIST AND COUNT THE NUMBER OF ATOMS FOR EVERY ELEMENT BELOW. C₆H₁₂O₆ H₂O H₂O₂ CO₂ C= 6 H= 2 H= 2 C= 1 H= 12 +O= 1 +O= 2 +O= 2 3 atoms 4 atoms 3 atoms +_O= 6_ 24 atoms 3. HOW MANY TOTAL ELEMENTS and TOTAL ATOMS ARE IN EACH CHEMICAL EQUATION? C₆H₁₂O₆ H₂O H₂O₂ CO₂ 3 ELEMENTS 2 ELEMENTS 2 ELEMENTS 2 ELEMENTS C= 6 H=2 H= 2 C= 1 H= 12 +O= 1 +O= 2 +O= 2 +O= 6 3 4 3 24 4. LABEL THE REACTANTS AND PRODUCTS. ARE THE FOLLOWING EQUATIONS BALANCED? TO FIND OUT, COUNT THE ATOMS OF EACH ELEMENT ON EACH SIDE OF THE ARROW. P + O2 → P4O10 reactants products Mg + O2 → MgO reactants products P= 1 P= 4 O= 2 O= 10 NOT BALANCED Mg = 1 Mg = 1 O=2 O=1 NOT BALANCED 5. DRAW A MODEL WITH CIRCLES OF THE FOLLOWING COMPOUNDS. FILL IN THE CIRCLES TO REPRESENT H₂O. 6. TEARING PAPER IS AN EXAMPLE OF A ___PHYSICAL__ CHANGE. 7. BURNING PAPER IS AN EXAMPLE OF A __CHEMICAL__ CHANGE. 8. FREEZING, MELTING AND BOILING WATER ARE ALL EXAMPLES OF _PHYSICAL__ CHANGES. 9. EXAMPLES OF A _CHEMICAL_______ CHANGE ARE………………………. A. BUBBLES OR GAS IS FORMED B. COLOR CHANGES C. FORMING A PRECIPITATE (TWO LIQUIDS FORM A SOLID) D. WHAT ELSE COULD GO HERE? _ENERGY_________________ 10. THE LAW OF CONSERVATION OF ENERGY SAYS THAT CHEMICAL EQUATIONS MUST BE BALANCED. IF THERE ARE 2 OXYGENS ON THE LEFT SIDE WE MUST HAVE __2___OXYGENS ON THE RIGHT SIDE. WHICH MEANS MASS HAS BEEN CONSERVED OR SAVED. IF THERE ARE 2 OXYGEN ON THE LEFT, AND 4 OXYGEN ON THE RIGHT, IS THE EQUATION BALANCED? ___No, as the law of conservation of mass states that the mass must equal on both sides of the equation in order for it to be conserved (saved/same)________________ Explain why or why not in complete sentences. 11. C= 3 C= 1 H= 8 H= 2 O= 1 O= 1 + 2 = 3 C3H8 + 10O → 4H2O + 3CO2 NOT BALANCED 12. C= 1 C= 1 H= 4 H= 2 O= 2 O= 3 NOT BALANCED CH4 + 2O2 → C02 + 2 H20 DETERMINE IF THE ABOVE CHEMICAL EQUATIONS ARE BALANCED OR NOT. THEN BALANCE IT IF NEEDED. SHOW YOUR WORK in a separate sheet of paper. 13. COMPLETE YOUR C = unexpected Color E = Energy (light, sound, heat) G = Gas (bubbles) S = Solid formation (when two liquid combine and form a solid, also known as a precipitate)ACRONYM/NOTES.






