Water and its relation to Osmosis & Diffusion Living cells contain aqueous
advertisement

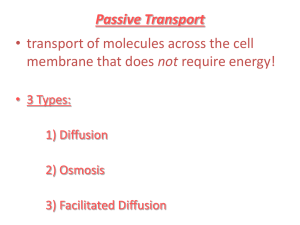
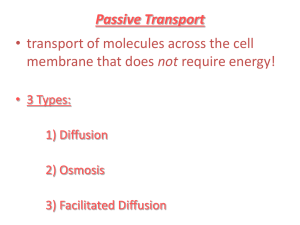
Water and its relation to Osmosis & Diffusion Living cells contain aqueous (water) solution and they are surrounded by aqueous solutions If the concentration of dissolved substances (solutes) is not the same inside and outside the cell – A process called osmosis occurs Osmosis: the movement of water molecules from an area with less solutes (low concentration) to an area with more solutes in the solution (high concentration) The water passes through the semi-permeable membrane until the concentration of the solutes is the same on both sides. Osmosis is the movement of water (red dots) through a semi permeable membrane to a higher concentration of solutes (blue dots). What are semi-permeable membranes? Those membranes that allow the solvent (liquid), but not the solutes to pass from one side of the membrane to another EX: egg, cellophane, and cell membranes In these membranes the pores are large enough for water to pass through but too small for other compounds to pass Examples Sailor died from dehydration due to the high concentration of salt in the seawater. After drinking the seawater the high concentration of salt caused the water to travel out of his cells. This caused the cells to dehydrate or shrink How do we get pickles? cells contain aqueous solutions – intracellular fluid The cells are also surrounded by an aqueous solutionintercellular fluid Cells function and survive by keeping the concentration within and around the cell approximately the same. Living If the concentration of solute in the fluid surrounding the cell is higher than inside the cell the solution is hypertonic Water will travel out of the cell through the membrane - crenation Red Blood Cells in a Hypertonic Solution Hypertonic Solution The word "HYPER" means more, in this case there are more solute (salt) molecules outside the cell, which causes the water to be sucked in that direction. If the concentration of the solute outside the cell is less than inside the cell it is called a hypotonic solution. Hemolysis will occur – additional water will travel into the cell causing it to swell and rupture. Red Blood Cells in a Hypotonic Solution Hypotonic Solution The word "HYPO" means less, in this case there are less solute (salt) molecules outside the cell, so water will move into the cell. When the fluids have the same concentrations they are called isotonic EX: when fluids are administered to a patient Red Blood Cells in an Isotonic Solution Isotonic Solution If the concentration of solute (salt) is equal on both sides, the water will move back in forth but it won't have any result on the overall amount of water on either side. "ISO" means the same What is Diffusion? Diffusion is the natural tendency of molecules to flow from higher concentrations to lower concentrations The rate of diffusion depends on the weight of the molecules—heavy molecules diffuse more slowly than light molecules. Diffusion of Gases A bottle of perfume opened in one corner of a room would slowly fill the room Molecules disperse from a concentrated source, the newly opened bottle, until they are evenly distributed throughout the room Smell of freshly baked cookies Diffusion of Liquids When two fluids mix together without any help from us. We can explain this using the idea that particles move. Instead of the particles moving around and mingling with particles of the same material, they move around and mingle with particles of the other material as well How Diffusion of Liquid in Liquid Works Add a drop of food coloring in a container of water. The color will “diffuse” or disperse throughout the entire sample until the sample is a uniform color. http://paer.rutgers.edu/pt3/movies/mixing2.mov How Diffusion of Solid in Liquid Works The solute (solid) dissolving in the solvent is also an example of diffusion. The water “attacks” the solute which eventually dissolve. What are ways that this process in increased? How Diffusion of Solid in Liquids Works Diffusion across a Membrane Selectively Permeable membranes – Living membranes alter what may pass through at any given time Diffusion of CO2 and O2 in the Lung Kidney Dialysis Review Osmosis: the movement of water molecules from an area of low solute concentration to an area of high solute concentration. The water passes through the semipermeable membrane until the concentration of the solutes is the same on both sides. Diffusion is the natural tendency of molecules to flow from higher concentrations to lower concentrations Movement may be of molecules or ions across a semi-permeable membrane Depends on molecular size, ionic charge, and molecular solubility (ability of solute to dissolve in solvent) Diffusion or Osmosis? 1% salt (low concentration) in the bag 10% salt (high concentration) surrounding the bag Which way does the water move? Diffusion or Osmosis? Iodine solution in the bag Starch solution surrounding the bag. What do you predict will happen? Lab Set-up