Ch. 8 Test Review
advertisement
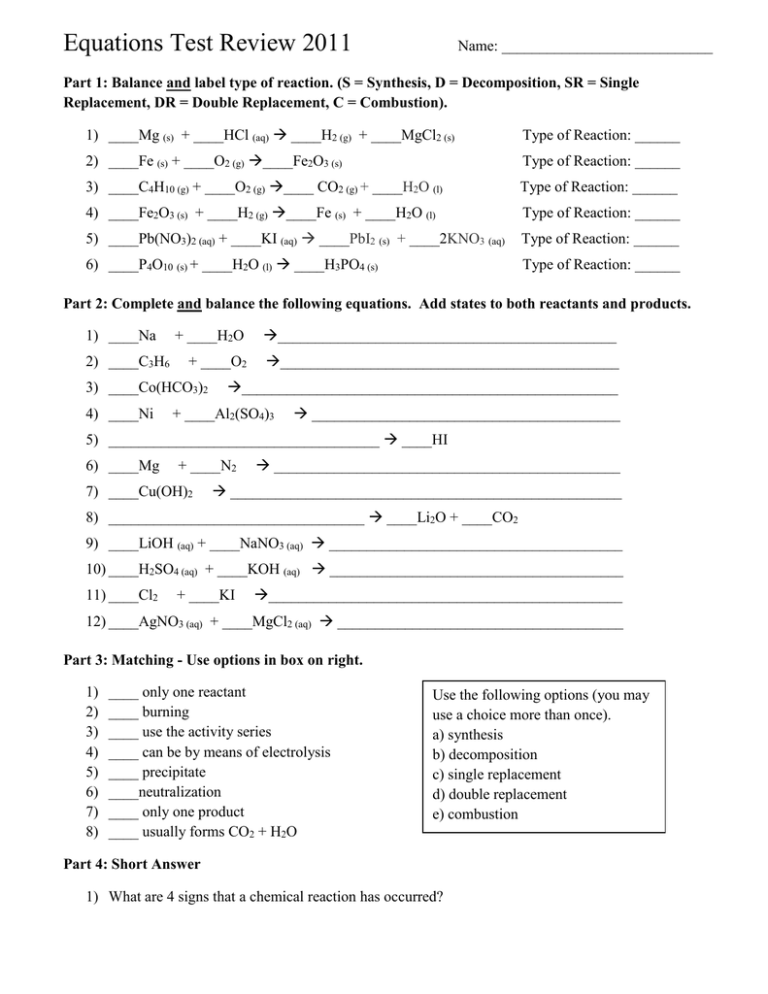
Equations Test Review 2011 Name: ____________________________ Part 1: Balance and label type of reaction. (S = Synthesis, D = Decomposition, SR = Single Replacement, DR = Double Replacement, C = Combustion). 1) ____Mg (s) + ____HCl (aq) ____H2 (g) + ____MgCl2 (s) Type of Reaction: ______ 2) ____Fe (s) + ____O2 (g) ____Fe2O3 (s) Type of Reaction: ______ 3) ____C4H10 (g) + ____O2 (g) ____ CO2 (g) + ____H2O (l) Type of Reaction: ______ 4) ____Fe2O3 (s) + ____H2 (g) ____Fe (s) + ____H2O (l) Type of Reaction: ______ 5) ____Pb(NO3)2 (aq) + ____KI (aq) ____PbI2 (s) + ____2KNO3 (aq) Type of Reaction: ______ 6) ____P4O10 (s) + ____H2O (l) ____H3PO4 (s) Type of Reaction: ______ Part 2: Complete and balance the following equations. Add states to both reactants and products. 1) ____Na 2) ____C3H6 + ____H2O _____________________________________________ + ____O2 _____________________________________________ 3) ____Co(HCO3)2 4) ____Ni __________________________________________________ + ____Al2(SO4)3 _________________________________________ 5) ____________________________________ ____HI 6) ____Mg + ____N2 7) ____Cu(OH)2 ______________________________________________ ____________________________________________________ 8) __________________________________ ____Li2O + ____CO2 9) ____LiOH (aq) + ____NaNO3 (aq) _______________________________________ 10) ____H2SO4 (aq) + ____KOH (aq) _______________________________________ 11) ____Cl2 + ____KI _______________________________________________ 12) ____AgNO3 (aq) + ____MgCl2 (aq) ______________________________________ Part 3: Matching - Use options in box on right. 1) 2) 3) 4) 5) 6) 7) 8) ____ only one reactant ____ burning ____ use the activity series ____ can be by means of electrolysis ____ precipitate ____neutralization ____ only one product ____ usually forms CO2 + H2O Use the following options (you may use a choice more than once). a) synthesis b) decomposition c) single replacement d) double replacement e) combustion Part 4: Short Answer 1) What are 4 signs that a chemical reaction has occurred? 2) How would you determine if a solid metal will replace a metal ion in single replacement reactions? 3) When would you write “No Reaction” after combining two aqueous salt solutions? 4) How does law of conservation of mass apply to reactions? 5) From the following choices, circle the element that would be a diatomic: Cl N F Ca Br Na H Part 5: Net Ionic Reactions Write the balanced equation, complete ionic equation, and net ionic equation for the reaction between ammonium sulfide (aq) and iron (II) nitrate (aq). Balanced Eqn: _________________________________________________________________________ Complete Ionic Equation: ________________________________________________________________ Net Ionic Equation: _____________________________________________________________________ Part 6: Lab Reactions/Demos 1) Write the balanced chemical equation for the decomposition of Nickel (II) Carbonate. What gas is produced? What test could be used to verify the type of gas produced? 2) Write the balanced chemical equation for the reaction of water and sulfur dioxide. What indicator could be used to determine if an acid or base is formed? What color should you see after adding the indicator to your product? 3) Write the balanced chemical equation for the reaction of copper (II) oxide with another compound to produce a base. What indicator could be used to determine if a base is actually formed? What color should you see after adding the indicator to your product? 4) Write the balance chemical equation for the reaction of solid zinc with sulfuric acid. What gas is produced? What test could be used to verify the type of gas produced?



