Atoms, Elements, Compunds and Mixtures Review

Atoms, Elements, Compounds and Mixtures Review
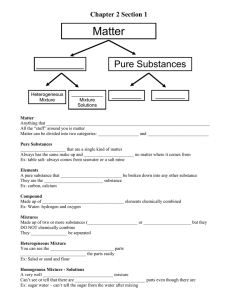
All matter is made up of
ATOMS.
Identify each labeled structure in the atom. Describe the charge for each particle.
A
B
A – Electron
Electrons have a negative charge.
B – Neutron
Neutrons have no charge (neutral).
C - Proton
Protons have a positive charge.
C
1. Where are protons and neutrons found in an atom?
In the nucleus of an atom.
2. Where are electrons found in an atom?
Outside the nucleus of an atom.
A
B
C
1. Identify structure A.
Support your answer.
Electron because it is negative.
2. Identify structure B.
Support your answer.
Proton because it is positive.
3. Identify structure C.
Support your answers.
Neutron because it is neutral. It does not have a charge.
A
4. Identify the structure in the middle of the atom that contains protons and neutrons.
Nucleus
B
C
5. Identify the atomic number of this atom. Support your answer.
Atomic number is 8 because the atom has 8 protons .
A positively charged particle is a
PROTON.
Which subatomic particle would be found outside the nucleus of an atom?
ELECTRON
Element or compound?
1. NaCl
2. O
Compound
Bc it is made up of 2 or more different elements.
Element
Bc it is made up of only one type of atom
3. CO
Compound
Bc it is made up of 2 or more different elements.
4. C
6
H
12
O
6
5. N
Compound Bc it is made up of 2 or more different elements.
Element Bc it is made up of only one type of atom
6. CO
7. H
2
2
O
Compound
Bc it is made up of 2 or more different elements.
Compound Bc it is made up of 2 or more different elements.
A chemical formula like CO
2 represents
1. an element 2. an atom
2. an electron 4. a compound
A substance made up of two or more elements that have been chemically combined is a
COMPOUND .
Which of the following is not an element?
1. oxygen (O)
2. sodium chloride (NaCl)
3. hydrogen (H)
4. Nitrogen (N)
A substance that cannot be changed into simpler substances by a chemical change is called a
(an)
1. element. 2. liquid.
3. solid. 4. mixture.
A substance made up of two or more elements that have been physcially combined is a
MIXTURE .
1. Explain why the substance on the left is a mixture.
It is made up of 2 or more substances PHYSICALLY
COMBINED.
2. Describe how a mixture is different than a compound.
A compound is made up of 2 or more substances CHEMICALLY
COMBINED .
Explain why sand and water is not a solution.
The sand did not dissolve in the water.
The sand was not evenly distributed.
A
B
Identify and describe the two parts of a solution.
Solute – what is dissolved
Solvent – What does the dissolving
used to separate the mixture.
Chromatography
2. Identify the type of mixture being separated.
Pigments
used to separate the mixture.
Filtration (filter paper)
2. Identify the type of mixture being separated.
Solid-liquid mixture
1. Identify the method used to separate the mixture.
Magnet
2. Identify the type of mixture being separated.
Mixture with iron filings
used to separate the mixture.
Evaporation
2. Identify the type of mixture being separated.
A solution
used to separate the mixture.
A sieve
2. Identify the type of mixture being separated.
Rocks and sand
Identify the 3 factors that can increase the solubility of a solute
(make the solute dissolve faster).
1.Increase the temperature of the solvent.
2.Use a smaller particle size.
3.Stirring
In sweetened tea, the sugar is called the
1. solute.
2. solvent.
3. colloid. 4. solution.
In sweetened tea, the water is called the
1. solute.
2. solvent.
3. colloid. 4. solution.
Describe the difference between a soluble and insoluble substance.
A soluble substance easily dissolves in a solvent.
An insoluble substance DOES NOT dissolve in a solvent.
Which of the following would help sugar dissolve faster in water?
1. stirring the water
2. decreasing the solubility of sugar
3. using larger particles of sugar
4. decreasing the water temperature
The substances in a mixture can be separated by physical means because
1. the substances are chemically combined.
2. the substances are chemically conded together.
3. the substances are physically, not chemically, mixed.
Sand and iron particles that are similar in size and color are mixed together in a beaker. What would be the best method of separating the particles?
1. Use tweezers to separate them.
2. Add water to the mixture.
3. Use a magnet to separate them.
4. Pour the mixture into a filter.
A green plant contains the green pigment chlorophyll. However, it also contains other pigments such as carotene and xanthophyll. How can these pigments be separated from each other?
1. Use tweezers to separate them.
2. Use filtration to separate them.
3. Use chromatography to separate them.
4. Use a sieve to separate them.
How can you separate sugar from a sugar water solution?
1. Use a magnet to separate the sugar from the water.
2. Use filtration to separate the sugar from the water.
3. Use chromatography to separate the sugar from the water.
4. Use evaporation to separate the sugar from the water.
How can you separate a solid from a liquid mixture?
1. Use a magnet to separate the insoluble substance from the mixture.
2. Use evaporation to separate the insoluble substance from the mixture
3. Use filtration to separate the insoluble substance from the mixture.
4. Use chromatography to separate the insoluble substance from the mixture.
Which statement is true regarding insoluble substances?
1. They can be easily dissolved in a solvent.
2. An example of an insoluble substance is ice tea dissolving in water.
3. Insoluble substances cannot dissolve in a solvent.
4. They can easily dissolve in a solute.
Which of the following is an example of a mixture of gases?
1. soft drink
2. air
3. oxygen
4. water
Which statement regarding mixtures is
true?
1. Mixtures consist of substances held together with chemical bonds.
2. Mixtures cannot be separated.
3. Mixtures consist of substances that are not chemically combined.
4. Mixtures do not include solutions.
The diagram represents a beaker containing a mixture of sugar, water, and sand. The sugar is
dissolved in the water, creating a
solution. The sand has settled to the bottom of the beaker.
1. How can the sand in the beaker be separated from the mixture?
Pour the mixture through filter paper. The sand will remain on the filter paper.
The sugar water solution will move through it.
2. Describe how the dissolved sugar can be separated from the sugar water solution.
Evaporation would remove the water from the beaker and leave the salt.
3. Explain why the sand and water is not a solution.
The sand is insoluble.
The sand did not dissolve in the water.
4. Explain why the substances in a mixture can be separated.
A mixture is made up of substances that are
PHYSICALLY mixed and not CHEMICALLY combined.
5. Identify the solute and solvent of the sugar water solution.
The sugar is the solute.
The water is the solvent.