novelsmall.ppt
advertisement

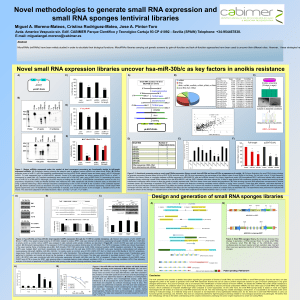
Novel small RNAs expression libraries uncover hsa-mir30c and hsa-mir30b as key factors in anoikis resistance Miguel A. Moreno-Mateos*, Verónica Barragán*, Belén Torres and José Antonio Pintor-Toro Avda. Americo Vespucio s/n. Edif. CABIMER Parque Cientifico y Tecnolgico Cartuja 93 CP 41092 - Sevilla (SPAIN) Telephone: +34954467838. E-mail: miguelangel.moreno@cabimer.es *These authors contribute equally to this work. -m ir1 9 l Le nt tro -c on Le nt -m ir1 5 tro Le nt -c on Le nt en g l ht l pC M Luc BS mir21(19,15) pLUC-BS 120 100 80 60 40 20 0 Lentcontrol Lent-19 Lentcontrol Luciferase mRNA levels Luciferase Activity Probe Y Ctr miR-19 Luc Plasmids 21 antisense Probe Y Ctr miR-19 21 sense Probe: pCMV-mir21 ll l Plasmids pCMV-control fu Lent-21 nt ro Lent-control V21 pLENT-DUAL pCMV-21 full lenght VCo pCMVcontrol -m ir2 1 Lent-21 pC M Lent-control l 20nt tro 30nt 120 100 80 60 40 20 0 Le nt mature miRNAs Luc -c on 40nt AAAA 180 160 140 120 100 80 60 40 20 0 Le nt AAAG 120 100 80 60 40 20 0 Lent-15 120 3’UTR PDCD4 100 80 60 140 40 120 Luciferase activity H1 E) D) Luciferase mRNA levels U6 C) Luciferase Activity B) Probe Y Ctr miR-21 Ctr miR-21 FL A) Probe Y Ctr miR-21 Ctr miR-21 FL INTRODUCTION During the past ten years, microRNAs (miRNAs) and other small non-coding RNAs (ncRNAs) have been deeply studied in order to elucidate their biological functions. At present, libraries to overexpress miRNAs are generated by synthesis and cloning of individual miRNAs. In this work, a patented methodology has been developed to generate a lentiviral expression library containing the whole population of small RNAs present into the cell including matures miRNAs. Using this library, a functional screening has been completed and several miRNAs involved in anoikis (cell death by loss of attachment) resistance identified. Among these, hsa-mir30c and hsa-mir30b were selected for further analysis because their expression strongly reduced cell death. Acquisition of anoikis resistance by these miRNAs was achieved through the inhibition of apoptotic pathways by downregulation of Caspase-3. 20 0 Lent-control Plasmids Lent-19 Lent-control Lent-15 Plasmids Probe: 19 sense 19 antisense 100 80 60 40 20 0 Lent-control Lent-21 pCMV control pCMV 21 Full Fig 1. Mature miRNAs expressed by dual convergent promoters are functionally similar to full length expressed miRNAs. A) Scheme showing the plasmid used to Lenght express mature miRNAs and other small RNAs (at the top) and the plasmid carrying the luciferase ORF followed by a binding site (BS) for miRNA 21, mir19 or mir15 Plasmids (at the bottom). B) RNAse protection assay of mature hsa-mir21 and hsa- mir19 overexpressed transiently by pLENT-DUAL plasmid. Hsa-mir21 full length (FL) was expressed from a pCMV-hsa-mir21 plasmid (Ctr:control; Y: Yeast RNA). C) Luciferase assay. Transient transfections were carried out using pLENT-DUAL (hsa-mir21, mir19 or mir15), pLuc-BS (mir21, mir19 or mir15) and pCMV-Renilla (as normalization control) at 100:10:1 proportions in HEK-293T cells. Hsa-mir21 Full length (FL) was expressed from a pCMV-hsa-mir21 plasmid. All transfections were performed in triplicate, and error bars represent standard error from three independent experiments. D) Quantification of Luciferase mRNA levels by Real time PCR in the conditions described in section C. Renilla mRNA was used as normalization control. E) Western blot analysis of Luciferase protein level and quantification in the conditions described in section C. At the bottom, Luciferase assay in a similar experiment described in section C. Transfections were carried out using pLENT-DUAL-hsa-mir21 and pCMV-hsa-mir21 full length and pLUC followed by 3’UTR of pdcd4 gene carrying a binding site of mir21. A) Small RNA isolation from MDA 231metastasic cell line 5’p Overexpreesion of full lenght hsa-mir30b OH 3’ T4 RNA ligase (no ATP) 3,5 3 2,5 2 1,5 1 0,5 0 Control 30b ddC 3’ 30c Cell Line T4 RNA ligase + ATP pSSFV ddC 3’ mir30c expression Independent clones selection Culture in attached conditions RT-PCR 20 Overexpreesion of full lenght hsa-mir30c Fold change 5’HO Anoikis screening (16 days in agar) OH 3’ ddC 3’ 5’p App mir30b expression pSSFV SubG1/SubG1 control (%) B) Fold change A) BbvII 120 100 80 60 40 20 0 Control 15 Control mir30b -PH 10 mir30c +PH 5 0 Control U6 30c Cell Line C CA T CAG GTA H1 30b Sequencing surviving clones B) pLENT-DUAL sRNA candidates C) Ctr 30b 30c CASP3 BIM Act 1 Ctr 30b 30c Pro-Casp3 +PH SubG1 (%) mir125b mir92a mir30c mir30b 1,2 -PH 0,8 0,6 0,4 0,2 Fig 2. A functional screening using a small RNAs expression library reveals hsamir30b and hsa-mir30c as important repressors of anoikis. A) Scheme of the small RNA cloning system to develop expression libraries based on pLENT-DUAL. B) Immortalized and sensitive to anoikis RPE1 cells were infected with a lentiviral library containing a small RNAs collection from MDA231 cell line. RPE1 infected cells were cultured for 16 days in agar dishes at low confluence avoiding any contact. Surviving clones were transferred to adhesive culture dishes to allow expand anoikis resistant cells. DNA from these clones was isolated and sequenced. C) Table showing small RNA sequences obtained from pLENT-DUAL of anoikis resistant clones. D) RPE1 cells were infected with lentivirus containing mature miRNA candidates or empty vector (control). Three days after infection, cells were deprived of serum for 24 hours and then were cultured in polyhema (PH) plates and metyl cellulose (2%) serum free medium for another 24 hours. Apoptosis was determined by FACS A) Active Casp3 3’UTR Casp3 (1570 pb) Mut2 1222-1228 Poorly conserved mir30b Cell Line mir30c SubG1 (%) 100 80 Control mir-30b mir-30c Control + Casp3 mir30b + Casp3 mir30c + Casp3 +PH 20 15 10 0 Control 30b -Trail 30c Control 30b 30c +Trail 40 Ctr 30b 30c 20 0 3'UTR MUT1 -PH Control 25 5 3'UTR WT Control + Casp3 Conclusions 30 60 50 45 40 35 30 25 20 15 10 5 0 Control Control 35 Luciferase activity Luc 30b 30c Fig 3. Full-length hsa-mir30b and hsa-mir30c downregulate anoikis and Caspase-3 expression. A) Levels of mature miRNAs expressed from a lentiviral plasmid carrying full length hsa-mir30b and full length hsa-mir30c-1 (empty vector as control). On the right, percentage of apoptosis in the infected cells. B) Western blot analysis of putative targets proteins of hsa-mir30b and hsa-mir30c (by TargetScan analysis) and related to anoikis. Active Caspase-3 was analysed in cells treated as described in section 2D. On the right, both protein and mRNA levels (measured by densitometry real time PCR respectively) of pro-Caspase-3 from three independent experiments. Actin was used as normalization control. C) Caspase-3 complementation. RPE1 cells were coinfected with two lentiviruses expressing miRNAs and cDNA of Caspase-3. Western blot analysis of Pro-Caspase-3, active Caspase-3 and Actin is shown. At the bottom, apoptosis determined by FACS of Caspase-3 infected cells growing in normal conditions or as described in section 2D. 120 Mut1 0 Act B) 1187-1193 Conserved Ctr mRNA Casp3 mir27a TCACAAGCTAGGGTCTCAGGGA GCGGAACTTAGACACTGTGAA Ctr Active Casp3 p53 mir23b mir125b mir27 Ctr 30b 30c mir23a TCAACATCAGTCTGATAAGCTT TGGAAATCCCTGGCAATGTGAT TTGGAAATCCC TGGCAATGTGAT ACAGGCCGGGACAAGTGCAATA GCTGAGAGTGTAGGATGTTTACA 30b 30c Pro-Casp3 mir21 mir21 mir23a mir23a mir92a mir30c Ctr CASP3 Act Fold change F11 TCAACATCAGTCTGATAAGCTA TTACTAGACTGTGAGCTCCT CGA AGTGGTAATCCCTGGCAATGTGAT TCAGCATCAGTCTGATAAGCTA AGCTGAGTGTAGGATGTTTACG 80 70 60 50 40 30 20 10 0 control B1 B2 B3 D4 F3 F7 mir21 mir151 mir23b mir21 mir30b Act control Clon small RNA sequence A8 Ctr D ) SubG1(%) C ) +PH -PH 3'UTR MUT2 Fig 4. Hsa-mir30b and hsa-mir30c downregulate Casp3 through its 3’ UTR. A) The 3’UTR of Caspase-3 mRNA was cloned downstream of Luciferase open reading frame in the pLUC-BS plasmid. The 3’UTR of Caspase-3 contains two putative miRNA30b/30c regulatory elements (MRE); one of them is highly conserved and the other one poorly conserved (by TargetScan analysis). B) HEK-293T cells were transiently contransfected with plasmids overexpressing miRNAs (hsa-mir30b, hsamir30c or control), pLUC-3’UTR casp3 (3’UTR WT) and pCMV-Renilla at 100:10:1 proportions. The same experiment was carried out using two mutant versions of pLUC-3’UTR casp3, where six nucleotides were changed both in the conserved (Mut 1) and poorly conserved (Mut 2) sites. Fig 5. Hsa-mir30b and Pro-Casp3 hsa-mir30c reduce cell death induced Act by TRAIL in MCF10A Cells. Cells were infected with lentivirus containing full-length hsa-mir30b and hsamir30c. Four days after infection, cells were cultured in presence of Trail (500 ng/ml) for seven hours. Apoptosis was determined by FACS. At the bottom, western blot analysis of Pro-Caspase-3 in these infected cells. Actin was used as control. - An expression library of the complete population of the cellular small RNAs has been performed. - Mature miRNAs expressed by this system and full length expressed miRNAs prove a similar functionality - Using this library, hsa-mir30b/c have been identified as important negative modulators of anoikis. - Caspase-3 is downregulated by hsa-mir30b/c through translational repression. Hsa-mir30b targets 3’UTR conserved MRE and hsa-mir30c targets both conserved and non-conserved MRE. - Complementation experiments suggest that hsa-mir30b/c decrease anoikis mainly targeting Caspase-3. - Other forms of Casp3-dependent cell death are also downregulated by hsa-mir30b/c. *This work is supported by grants from the Spanish MEC and the DGUI of the Junta de Andalucía.