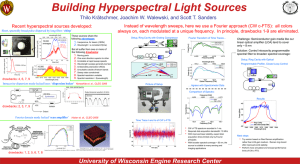
Hydrogen Spectral Lines Visible to Human Beings
advertisement

Physics 142 Homework Name Due in Recitation Reci. Section time: You may earn up to 10 points based on your work. Hydrogen Spectral Lines Visible to Human Beings 1. Solve the necessary equations in algebraic form for the quantum numbers of the electron states in hydrogen that emit visible photons. Use the integer n for the lower state and the integer m for the higher state. Use the symbol min for the shortest wavelength you can see and max for the longest wavelength you can see. 2. Now put in the numbers for min (380 nm) and for max (770 nm) and make a numerical table of all the visible lines of hydrogen. Higher state, m value Lower state, n value Transition Energy (eV) Wavelength (nm) (If you need more space for writing please write on the back of this page)