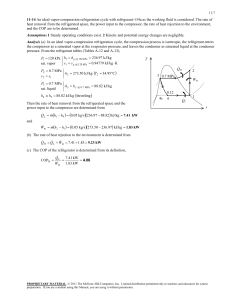
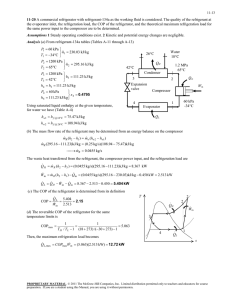
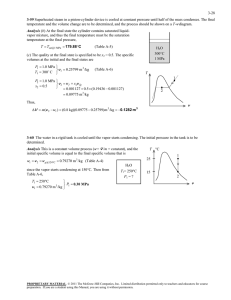
A refrigerator with refrigerant-134a as the working fluid is considered.... ME470 Refrigeration Cycles HW Solutions Inst: ... Chapter 11, Solution 18.
advertisement
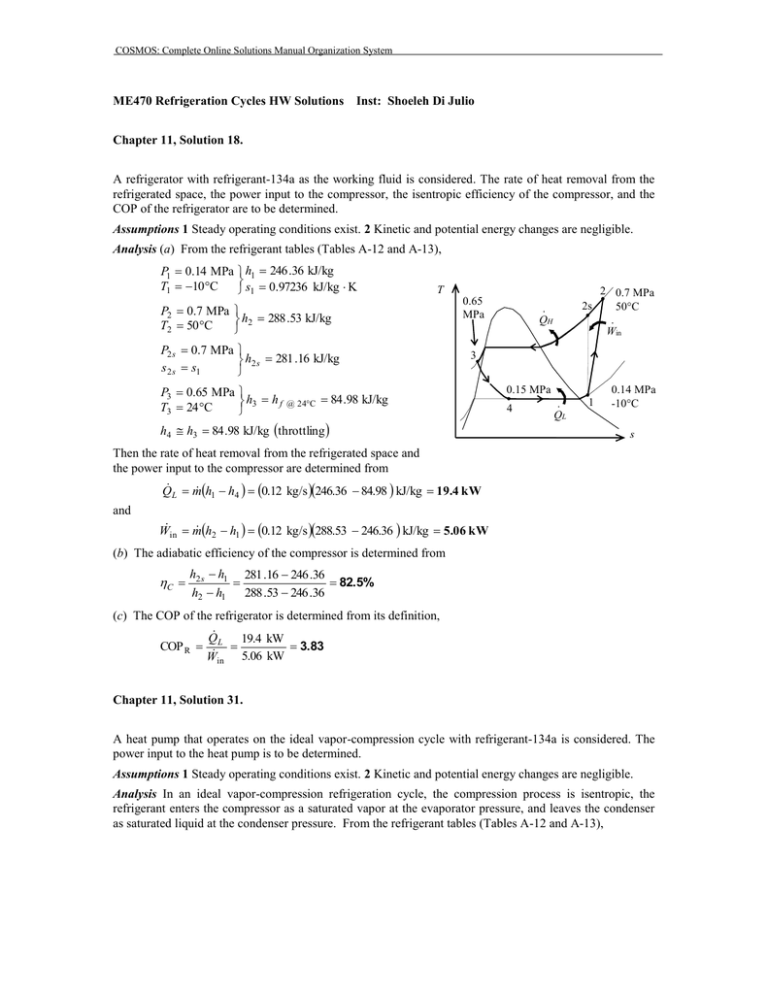
COSMOS: Complete Online Solutions Manual Organization System ME470 Refrigeration Cycles HW Solutions Inst: Shoeleh Di Julio Chapter 11, Solution 18. A refrigerator with refrigerant-134a as the working fluid is considered. The rate of heat removal from the refrigerated space, the power input to the compressor, the isentropic efficiency of the compressor, and the COP of the refrigerator are to be determined. Assumptions 1 Steady operating conditions exist. 2 Kinetic and potential energy changes are negligible. Analysis (a) From the refrigerant tables (Tables A-12 and A-13), P1 0.14 MPa h1 246 .36 kJ/kg s 0.97236 kJ/kg K T1 10 C 1 T P2 0.7 MPa h2 288 .53 kJ/kg T2 50 C P2 s 0.7 MPa h2 s 281 .16 kJ/kg s 2 s s1 P3 0.65 MPa h3 h f T3 24 C @ 24C 0.65 MPa 24C 2s · QH 2 0.7 MPa 50C · Win 3 84 .98 kJ/kg h4 h3 84 .98 kJ/kg throttling 0.15 MPa 4 · QL 1 0.14 MPa -10C s Then the rate of heat removal from the refrigerated space and the power input to the compressor are determined from Q L m h1 h4 0.12 kg/s 246.36 84.98 kJ/kg 19.4 kW and W in m h2 h1 0.12 kg/s 288.53 246.36 kJ/kg 5.06 kW (b) The adiabatic efficiency of the compressor is determined from C h2 s h1 281 .16 246 .36 82.5% h2 h1 288 .53 246 .36 (c) The COP of the refrigerator is determined from its definition, Q 19.4 kW COP R L 3.83 W in 5.06 kW Chapter 11, Solution 31. A heat pump that operates on the ideal vapor-compression cycle with refrigerant-134a is considered. The power input to the heat pump is to be determined. Assumptions 1 Steady operating conditions exist. 2 Kinetic and potential energy changes are negligible. Analysis In an ideal vapor-compression refrigeration cycle, the compression process is isentropic, the refrigerant enters the compressor as a saturated vapor at the evaporator pressure, and leaves the condenser as saturated liquid at the condenser pressure. From the refrigerant tables (Tables A-12 and A-13), COSMOS: Complete Online Solutions Manual Organization System P1 320 kPa h1 h g @320 kPa 251 .88 kJ/kg s s sat. vapor g @ 320 kPa 0.93006 kJ/kg K 1 P2 1.4 MPa s 2 s1 T h2 282 .54 kJ/kg P3 1.4 MPa h3 h f sat. liquid h4 h3 127 .22 kJ/kg @ 1.4 MPa House · QH 2 3 1.4 MPa 127 .22 kJ/kg throttling · Win 0.32 MPa The heating load of this heat pump is determined from 1 · QL 4 Q H m cT2 T1 water s 0.12 kg/s 4.18 kJ/kg C45 15 C 15 .05 kW and m R Q H Q H 15.05 kJ/s 0.09688 kg/s qH h2 h3 282.54 127.22 kJ/kg Then, W in m R h2 h1 0.09688 kg/s 282.54 251.88 kJ/kg 2.97 kW Chapter 11, Solution 32. A heat pump with refrigerant-134a as the working fluid heats a house by using underground water as the heat source. The power input to the heat pump, the rate of heat absorption from the water, and the increase in electric power input if an electric resistance heater is used instead of a heat pump are to be determined. Assumptions 1 Steady operating conditions exist. 2 Kinetic and potential energy changes are negligible. Analysis (a) From the refrigerant tables (Tables A-12 and A-13), P1 280 kPa h1 250 .83 kJ/kg T1 0C P2 1.0 MPa h2 293 .38 kJ/kg T2 60 C P3 1.0 MPa h3 h f T3 30 C @ 30C T 60C · QH · Win 30C 1 MPa 3 93 .58 kJ/kg h4 h3 93 .58 kJ/kg throttling 0.28 MPa 4 The mass flow rate of the refrigerant is Q Q H 60,000/3,6 00 kJ/s m R H 0.08341 kg/s qH h2 h3 293.38 93.58 kJ/kg Then the power input to the compressor becomes h2 h1 0.08341kg/s293.38 250.83 kJ/kg 3.55 kW Win m (b) The rate of hat absorption from the water is 2 House · QL 1 0C Water, 8C s COSMOS: Complete Online Solutions Manual Organization System h1 h4 0.08341kg/s250.83 93.58 kJ/kg 13.12 kW Q L m (c) The electrical power required without the heat pump is W e Q H 60 ,000 / 3600 kJ/s 16.67 kW Thus, W increase W e W in 16 .67 3.55 13.12 kW Chapter 11, Solution 42. A two-stage cascade refrigeration system is considered. Each stage operates on the ideal vapor-compression cycle with refrigerant-134a as the working fluid. The mass flow rate of refrigerant through the lower cycle, the rate of heat removal from the refrigerated space, the power input to the compressor, and the COP of this cascade refrigerator are to be determined. Assumptions 1 Steady operating conditions exist. 2 Kinetic and potential energy changes are negligible. 3 The heat exchanger is adiabatic. Analysis (a) Each stage of the cascade refrigeration cycle is said to operate on the ideal vapor compression refrigeration cycle. Thus the compression process is isentropic, and the refrigerant enters the compressor as a saturated vapor at the evaporator pressure. Also, the refrigerant leaves the condenser as a saturated liquid at the condenser pressure. The enthalpies of the refrigerant at all 8 states are determined from the refrigerant tables (Tables A-11, A-12, and A-13) to be h1 239 .16 kJ/kg , h2 260 .58 kJ/kg h3 63 .94 kJ/kg , h4 63 .94 kJ/kg h5 255 .55 kJ/kg , h6 269 .91 kJ/kg h7 95 .47 kJ/kg, T 0.8 MPa h8 95 .47 kJ/kg The mass flow rate of the refrigerant through the lower cycle is determined from an energy balance on the heat exchanger: E in E out E system0 (steady) 0 E in E out 6 0.4 MPa 7 3 8 2 A 5 B 4 · QL m h m h e e 0.14 MPa 1 s i i m A h5 h8 m B h2 h3 m B h5 h8 255 .55 95 .47 0.24 kg/s 0.1954 kg/s m A h2 h3 260 .58 63 .94 (b) The rate of heat removed by a cascade cycle is the rate of heat absorption in the evaporator of the lowest stage. The power input to a cascade cycle is the sum of the power inputs to all of the compressors: Q L m B h1 h4 0.1954 kg/s 239.16 63.94 kJ/kg 34.24 kW W in W compI, in W compII, in m A h6 h5 m B h2 h1 0.24 kg/s 269.91 255.55 kJ/kg 0.1954 kg/s 260.58 239.16 kJ/kg 7.63 kW (c) The COP of this refrigeration system is determined from its definition, Q L 34.24 kW COP R 4.49 7.63 kW Wnet,in COSMOS: Complete Online Solutions Manual Organization System COP R Q L 34.24 kW 4.49 7.63 kW Wnet,in Chapter 11, Solution 55. An ideal-gas refrigeration cycle with air as the working fluid is considered. The maximum and minimum temperatures in the cycle, the COP, and the rate of refrigeration are to be determined. Assumptions 1 Steady operating conditions exist. 2 Air is an ideal gas with variable specific heats. 3 Kinetic and potential energy changes are negligible. Analysis (a) We assume both the turbine and the compressor to be isentropic, the turbine inlet temperature to be the temperature of the surroundings, and the compressor inlet temperature to be the temperature of the refrigerated space. From the air table (Table A-17), T1 250 K T1 300 K h1 250.05 kJ / kg Pr 1 0.7329 h3 30019 . kJ / kg Pr 3 1.386 T · QH 27C -23C 3 Thus, Pr2 P2 Pr 30.7329 2.1987 T2 Tmax 342.2 K P1 1 h2 342 .60 kJ/kg Pr4 P4 1 Pr 1.386 0.462 T4 Tmin 219.0 K P3 3 3 h4 218 .97 kJ/kg 2 4 1 · QRefrig s (b) The COP of this ideal gas refrigeration cycle is determined from COPR qL wnet, in qL wcomp, in w turb, out where q L h1 h4 250.05 218.97 31.08 kJ / kg wcomp, in h2 h1 342.60 250.05 92.55 kJ / kg wturb, out h3 h4 30019 . 218.97 81.22 kJ / kg Thus, COPR 31.08 2.74 92.55 81.22 (c) The rate of refrigeration is determined to be Q refrig m qL 0.08 kg/s31.08 kJ/kg 2.49 kJ/s Chapter 11, Solution 61. An ideal-gas refrigeration cycle with air as the working fluid is considered. The lowest temperature that can be obtained by this cycle, the COP, and the mass flow rate of air are to be determined. Assumptions 1 Steady operating conditions exist. 2 Air is an ideal gas with constant specific heats. 3 Kinetic and potential energy changes are negligible. COSMOS: Complete Online Solutions Manual Organization System Properties The properties of air at room temperature are cp = 1.005 kJ/kg·K and k = 1.4 (Table A-2). Analysis (a) The lowest temperature in the cycle occurs at the turbine exit. From the isentropic relations, P T2 T1 2 P1 k 1 / k P T5 T4 5 P4 266 K 4 0.4 / 1.4 395 .3 K 122.3 C k 1 / k 1 258 K 4 0.4 / 1.4 173 .6 K 99.4C Tmin (b) From an energy balance on the regenerator, E in E out E system0 (steady) 0 E in E out m e he m i hi m h3 h4 m h1 h6 T · QH 3 27C -7C -15C 2 Qrege 4 5 1 n 6 · QRefrig or, s m c p T3 T4 m c p T1 T6 T3 T4 T1 T6 or, T6 T1 T3 T4 7C 27 C 15 C 49 C Then the COP of this ideal gas refrigeration cycle is determined from COP R qL qL wnet,in wcomp, in w turb,out h 6 h5 h2 h1 h4 h5 T6 T5 T2 T1 T4 T5 49 C 99 .4C 1.12 122 .3 7 C 15 99 .4C (c) The mass flow rate is determined from Q refrig Q refrig Q refrig 12 kJ/s m 0.237 kg/s qL h6 h5 c p T6 T5 1.005 kJ/kg C 49 99.4 C Chapter 11, Solution 71. The conditions at which an absorption refrigeration system operates are specified. The maximum COP this absorption refrigeration system can have is to be determined. Analysis The maximum COP that this refrigeration system can have is T COP R, max 1 0 Ts TL T T L 0 298 K 273 1 393 K 298 273 2.64 COSMOS: Complete Online Solutions Manual Organization System