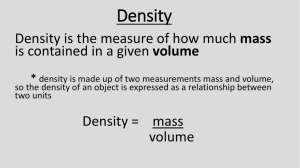1305-Home-work-2.doc
advertisement

CHEM 1305- Home-work 2 NAME: 3 1) Which of the following units of measure is equivalent to a cm (cubic centimeter)? A) mL B) mm C) mg D) gr 2) A cylindrical slug of copper has a diameter of 68.3 mm and a height of 0.120 m. What is the volume of the cylinder of copper in cm3? (cylinder volume: V = r2h) A) 702 cm3 B) 18.2 cm3 C) 176 cm3 D) 1460 cm3 E) 4.40 102 cm3 3) Which of the following unit conversion factors would change g/mL to lb/ft3? A) g cm3 in 3 ft 3 lb mL mL cm3 in 3 g B) g mL cm3 g in 3 3 3 3 mL cm lb ft in C) g lb mL cm in 3 mL g cm in ft 3 D) g mL cm3 lb in 3 3 3 3 mL cm g ft in 4) 4.34 quarts is equivalent to how many microliters? (1 quart = 946mL) A) 4.11 103 L B) 124 L C) 2.69 L D) 6.74 105 L E) 4.11 106 L 5) What is the mass of 36.0 mL of a liquid if its density is 2.70 g/mL? A) 13.3 g B) 0.0750 g C) 16.3 g D) 0.748 g E) 97.2 g 6) If object A weighs 24 grams and has a volume of 3 mL and object B weighs 14 g and has a volume of 7 mL, ________. A) B is more dense than A B) A and B have equal densities C) B is half as dense as A D) A is four times as dense as B 7) Density is an easy way to distinguish between gold (density: approx. 19 g/cm3), fool’s gold (density: approx. 4.5 g/cm3), and sand (density: approx. 2.5 g/cm3). If a large golden rock has a mass of 252 g and a volume of 56 cm3, which type of mineral is it? A) gold B) fool’s gold C) sand 8) In a laboratory class with 80 students, 50% of the students are male, and 10% of the male students are left handed. How many students in the laboratory are male students that are left handed? A) 3 student B) 8 students C) 10 students D) 4 students E) 2 students 9) The temperature of an average lightning bolt is 3.0 104 °C. What is the equivalent temperature on the Fahrenheit scale? A) 6.9 105 °F B) 1.7 04 °F C) 3.8 103 °F D) 2.0 105 °F E) 5.4 104 °F 10) A temperature of 354 on the Kelvin scale is equivalent to what temperature on the Fahrenheit scale? Perform the following conversions. Be sure to use proper significant figures in your answer. You may use these conversion factors to help you. 1.61 km = 1 mile 2.54 cm = 1 in 1.06 qt = 1.0 L 454 g = 1 lb 1 gal = 4gt 11) 86.7 km to miles 12) 65 in to mm 13) 82 nL to GL 14) 310 lb. to kg 15) 26.9 square centimeters is equivalent to how many square feet? A) 117 ft2 16) B) 0.0290 ft2 C) 3.67 ft2 D) 0.00144 ft2 E) 0.186 ft2 The coast guard seized 175 hectograms of cocaine on a Columbian boat they boarded off the coast of Florida. What is the mass of the cocaine seized in grams? A) 1750 grams B) 0.1759 grams C) 17500 grams 17) Which of these samples has the smallest mass? D) 175000 grams E) 0.001750 grams A) 1.6 x 102 g B) 1.6 x 10-2 g C) 1.6 x 10-4 mg D) 1.6 x 10-7 kg 18) The typical volume of an aluminum can of soda is 355 mL. What is the equivalent volume in gallons? A) 3.37 105 gal B) 0.0939 gal C) 5.75 gal D) 0.673 gal E) 1.23 gal 19) Automotive batteries generally are filled with sulfuric acid. If a battery has a volume of 1.86 L and contains 3.42 103 grams of sulfuric acid, what is the density of sulfuric acid in g/mL? A) 5.43 10-4 g/mL B) 5.84 103 g/mL D) 19.85 g/mL E) 0.846 g/mL C) 1.84 g/mL 20) A certain children’s fever reducing medicine has a concentration of 250 mg/10 mL. If a child is to receive 2 teaspoons of this medicine, how many mg of medicine is being received in one dose? (1 tsp = 5 mL) A) 650 mg B) 275 mg C) 188 mg D) 250 mg E) 510 mg