Practice Problems 1405 Chapter 5.doc
advertisement
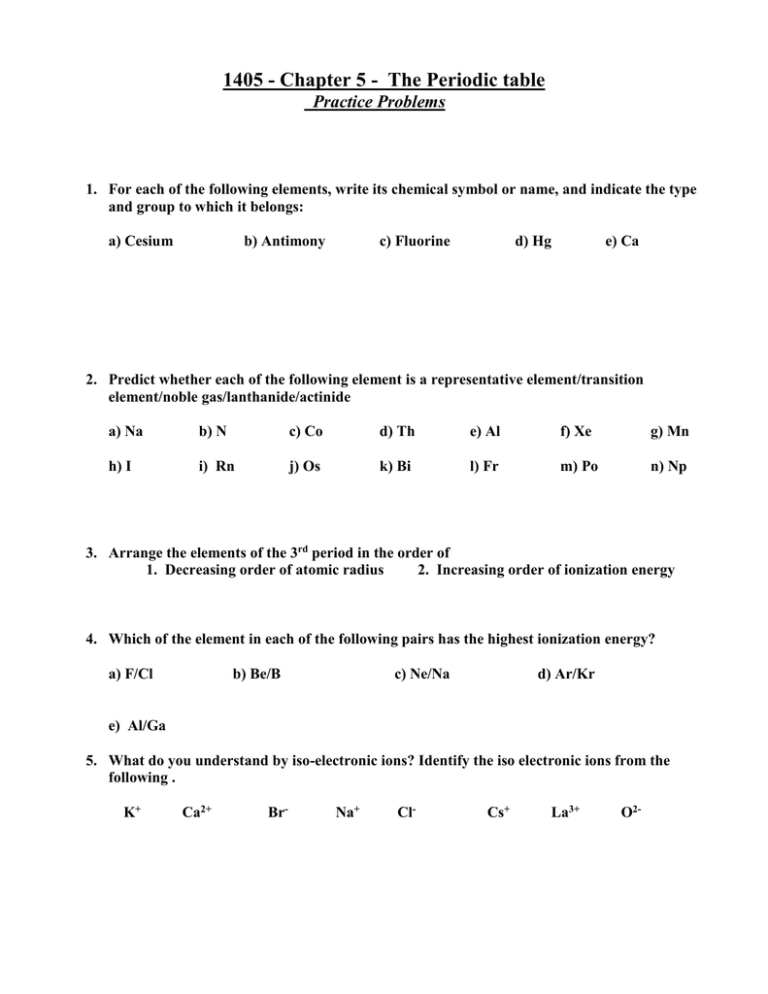
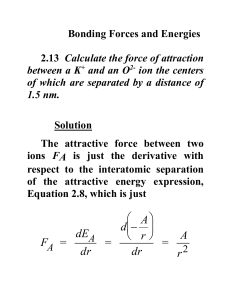
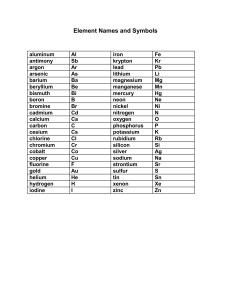
1405 - Chapter 5 - The Periodic table Practice Problems 1. For each of the following elements, write its chemical symbol or name, and indicate the type and group to which it belongs: a) Cesium b) Antimony c) Fluorine d) Hg e) Ca 2. Predict whether each of the following element is a representative element/transition element/noble gas/lanthanide/actinide a) Na b) N c) Co d) Th e) Al f) Xe g) Mn h) I i) Rn j) Os k) Bi l) Fr m) Po n) Np 3. Arrange the elements of the 3rd period in the order of 1. Decreasing order of atomic radius 2. Increasing order of ionization energy 4. Which of the element in each of the following pairs has the highest ionization energy? a) F/Cl b) Be/B c) Ne/Na d) Ar/Kr e) Al/Ga 5. What do you understand by iso-electronic ions? Identify the iso electronic ions from the following . K+ Ca2+ Br- Na+ Cl- Cs+ La3+ O2- 6. Identify the element with greater metallic character K/Ca Sc/V Fe/Co Zn/Cd Pb/Bi Li/Na 7. Find the Lewis symbols of the following elements Al Si S Cl Ar Na+ F- 7. Predict the ionic charge of the following elements B S Sr Cs Sb Br I Xe