26WaterElectrolyteBalance
advertisement

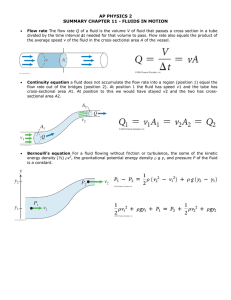
Anatomy & Physiology 34B Chapter 26 – Fluid, Electrolyte, and Acid-Base Balance I. Overview A. Cellular function requires a fluid medium with a homeostatic __________ of the following 1. ________, in which daily water intake and loss are equal 2. _______________, in which the amount of electrolytes absorbed by the small intestine balance the amount lost from the body, mainly through the urine 3. ________, in which the body rids itself of acid (H+ ions) at a rate that balances its metabolic production, maintaining a stable pH B. These balances are maintained by the ___ of the bodily systems, except for the reproductive system II. Fluid Balance A. A young, lean adult male body is about _______% water B. ______________________ in which water is found include 1. 65% in _______cellular fluid (____) within cells 2. 35% in _______cellular fluid (____), which includes a. 25% _____________ fluid around cells b. 8% blood _________ and lymph c. 2% _____cellular fluid, such as cerebrospinal, synovial, serous, vitreous & aqueous humors, bile, and fluids in the GI, urinary, and respiratory tracts C. Water moves by ________ from one compartment to another, so the ECF and ICF osmolarities rarely differ D. A person is in a state of water __________ when daily water intake and losses are equal 1. Water gains come from metabolic water from cellular respiration and dehydration synthesis, as well as from __________ water 2. Water is lost in _______, feces, expired breath, sweat, and transpiration through the skin E. Fluid intake is regulated by the thirst center in the _____________ 1. The hypothalamus responds to signs of ______________, such as a. __________ II, produced in response to falling blood pressure b. ______, released in response to raising blood osmolarity c. Signals from _____________ neurons in the hypothalamus that monitor blood osmolarity 2. ___________ is stimulated and satiated by a. The thirst center sends sympathetic signals to the ___________ glands to inhibit salivation b. Short term satiation of thirst is accomplished by __________ and moistening the mouth and inflating the stomach b. Long term satiation of thirst depends on ____________ water from the small intestine and lowering blood osmolarity F. Fluid output is ____________ by factors that control urine output 1. As __________ is reabsorbed or excreted, water accompanies it 2. _____ is secreted by the posterior pituitary gland in response to dehydration, which causes the kidneys to reabsorb water 2 3. ADH secretion is inhibited if blood volume or ___________ are too high, or if blood ______________ is too low G. _____________ of Water Balance occur if there is an abnormality of total fluid volume, concentration, or distribution among the compartments 1. Fluid _____________ arises when output exceeds intake over a period of time. Two kinds of fluid deficiency are a. Volume depletion (_______________), in which proportionate amounts of water and sodium are lost; may be due to hemorrhage, burns, vomiting, diarrhea, or hyposecretion of ____________ b. _________________ (negative water balance), in which volume is reduced and osmolarity is elevated because the body has lost more water than sodium; caused by lack of drinking water, diabetes mellitus, ______ hyposecretion, profuse sweating c. Severe fluid deficiency can result in circulatory shock and _____ 2. Fluid __________ can occur in two forms a. ___________ excess - the retention of excess fluid with normal osmolarity; can result from ___________ hypersecretion or renal failure b. ______________ hydration (water intoxication) – the retention of more water than sodium, reducing osmolarity, as when one loses water and sodium in sweat and drinks plain water; can cause pulmonary and cerebral _______ 3. Fluid ________________ – total body water may be normal, but fluid accumulates in a particular location; occurs in edema, hemorrhage, and pleural _________ where fluids accumulate in the pleural cavity III. Electrolyte Balance A. Electrolyte (____) functions include 1. Provide enzyme ___________ (e.g., Zn2+, Mg2+) 2. Allow action potentials in neurons and muscles (___, ___, Cl-) 3. Stimulate the secretion and action of ___________ and neurotransmitters (e.g., Ca2+ ) 4. Allow muscle contraction (___) 5. Maintain acid-base balance (___, HCO3-, and phosphates) 6. Allow secondary active transport across membranes (___, ___) 7. Stimulate __________ across cell membranes (Na+) B. Major _________ include Na+, K+, Ca2+, and H+ C. Major ________ are Cl-, HCO3- , phosphates (HPO42- and H2PO4-), and proteins D. Sodium (Na+) is the main cation in the ______ 1. Na+ _____________ include a. ____________ and fluid balance b. _________ and muscle activity c. ______________ of molecules (e.g., glucose) across cell membranes d. Acid-base ______________ (NaHCO3) e. ________ generation via the Na+/K+ pump 2. Sodium _________________ is maintained by a. Aldosterone promotes Na+ ______________ b. ADH reduces Na+ concentration by promoting water _____________ independently of Na+ 3 c. Atrial naturietic peptide inhibits Na+ and water reabsorption, ____________ blood pressure 3. _________________ of sodium include a. Hypernatremia is an excess of Na+, which causes water retention, _______________, and edema b. Hyponatremia is a deficiency of Na+, often a result of __________ hydration E. Potassium (K+) is the major cation in the ____. It is important for the same reasons as Na+ and is a cofactor for some enzymes 1.Potassium homeostasis is maintained mainly by ____________, which promotes excess K+ excretion by the kidneys 2. _______________ of potassium include a. Hyperkalemia causes _______ and muscle dysfunction, and can cause cardiac arrest b. Hypokalemia ____________ nerve and muscle function F. Chloride (Cl-) is the major anion of the _____ 1. Chloride _____________ include a. Regulation of ___________ balance (NaCl) b. Formation of stomach acid (_____) c. The chloride _______ mechanism in respiratory and renal function 2. Chloride homeostasis follows ____ and other cations, and is regulated as a side effect of Na+ homeostasis 3. The primary effect of chloride imbalances is a ___ imbalance G. Calcium (Ca2+) has low intracellular concentrations, but is often sequestered in smooth ______, then released when needed 1. Calcium is necessary for a. Muscle _____________ b. Nerve transmission and ____________ of neurotransmitters c. Blood ____________ d. A second ______________ for some hormone actions e. ________ and tooth formation 2. Calcium homeostasis is regulated by a. _______________ hormone (PTH) – increases serum Ca2+ levels by bone reabsorption and intestinal uptake b. Calcitriol (vit. __) is required for intestinal uptake of Ca2+ c. _____________ – decreases serum Ca2+ levels and increases bone deposition 3. Hypercalcemia can result from acidosis, _____parathyroidism, or ____thyroidism; causes muscle weakness, depressed reflexes, and cardiac _________________ 4. Hypocalcemia can result from alkalosis, vit. __ deficiency, diarrhea, pregnancy, lactation, ______parathyroidism, or _____thyroidism; causes potentially fatal muscle ________ H. Phosphates (PO43-, HPO42-, H2PO4-) are relatively concentrated in the _____, where they are generated by _____ hydrolysis 1. Phosphate __________ include a. Phosphates are a component of nucleic acids, ________lipids, ATP, GTP, cAMP, and related compounds b. Phosphates activate many metabolic pathways by ______________ substances such as glucose and enzymes 4 c. Phosphates are important acid-base _________ 2. Phosphate levels are regulated by ________________ hormone, which increases phosphate excretion and minimizes the formation of CaPO4 3.Phosphate imbalances are not as __________ as other electrolyte imbalances IV. Acid-Base Balance A. The pH of the ECF is normally maintained between _____-___, despite constant production of acidic products (e.g., lactic acid, phosphoric acids, fatty acids, carbonic acid) B. Acids, Bases, & Buffers 1. An _______ is any chemical that releases H+ ions in solution a. _______ acids (____) give up most of their H+ ions and can ________ pH significantly b. _______ acids (H2CO3) do not give up many ___ ions, thus affect pH only slightly 2. A _______ is any chemical that takes up H+ ions in solution a. _______ bases (____) have a strong tendency to bind H+ ions and _______ pH b. ______ bases (HCO3-) bind less ___, thus have less of an effect on pH 3. A _________ is any mechanism that resists changes in pH by converting strong acids or bases to weak ones. The body has both physiological and chemical buffers a. Physiological buffers, such as the __________ and _______ systems, stabilize pH by controlling the body’s output of acids, bases, or CO2 b. ___________ buffers bind ___ and remove it from solution as its concentration begins to rise, or release ___ into solution as its concentration falls. 4. Three ____________ buffer systems in the body are the a. _____________ buffer system, represented by the eqn.: CO2 + H2O H2CO3 HCO3- + H+, and has an optimal pH of ____ 1) The lungs and kidneys remove ____, which keeps the rxn. moving to the ______, reducing H+ ions 2) If there is a need to lower pH, the kidneys excrete ______, which moves the rxn. to the ________, increasing the H+ concentration b. ___________ buffer system has an optimal pH of ___, and is important for buffering the renal _______ & ICF. The rxn. is H2PO4- HPO42- + H+ c. ___________ buffer system accounts for ___ of all chemical buffering in body fluids, due to side groups of _______ acids 1) Carboxylic acid groups (____) release H+ when ph begins to rise 2) Amino groups (____) bind H+ when pH falls too low C. __________ control of pH - the respiratory system buffers pH by adjusting pulmonary ___________ 1. ________ ventilation allows ____ to accumulate in the blood and lower its pH by the rxn. CO2 + H2O H2CO3 HCO3- + H+ 2. _________ ventilation expels ____, reversing the above rxn, lowering H+, and ___________ the pH 5 D. _______ control of pH – the kidneys neutralize ______ acid or base than any other buffer system in the body 1. They secrete ___ into the tubular fluid, where it binds to chemical buffers and is ____________ in the urine 2. The above H+ normally neutralizes all the ______ in the tubular fluid, making urine bicarbonate free 3. Excess H+ in the tubular fluid can be _________ by phosphate and ammonia (____) E. ______________ of acid-base balance 1. ___________ is a pH of _____ a. ___________ acidosis occurs when pulmonary gas exchange is insufficient to expel ____ as fast as the body produces it b. _____________ acidosis is the result of lactic acid or ketone accumulation, ingestion of acidic drugs, such as aspirin, or loss of base, as in __________ 2. ____________ is a pH of _____ a. Respiratory alkalosis results from hyper__________ b. __________ alkalosis is rare, but can be caused by overuse of antacids or loss of stomach acid through ___________ 3. ______________ acidosis or alkalosis is a pH imbalance that the body cannot correct on its own; it requires clinical intervention (i.e., ________ replacement therapy) 4. _____________ acidosis or alkalosis is an imbalance that the body’s homeostatic mechanisms can correct a. Respiratory compensation is correction of the pH through changes in pulmonary ___________ b. Renal compensation is correction of pH by changes in ___ secretion by the kidneys 5. Water, electrolyte, and _____-____ imbalances are intimately entwined; an imbalance area can cause or result from an imbalance in another