Request for Extracorporeal Photopheresis (ECP) for GvHD
advertisement
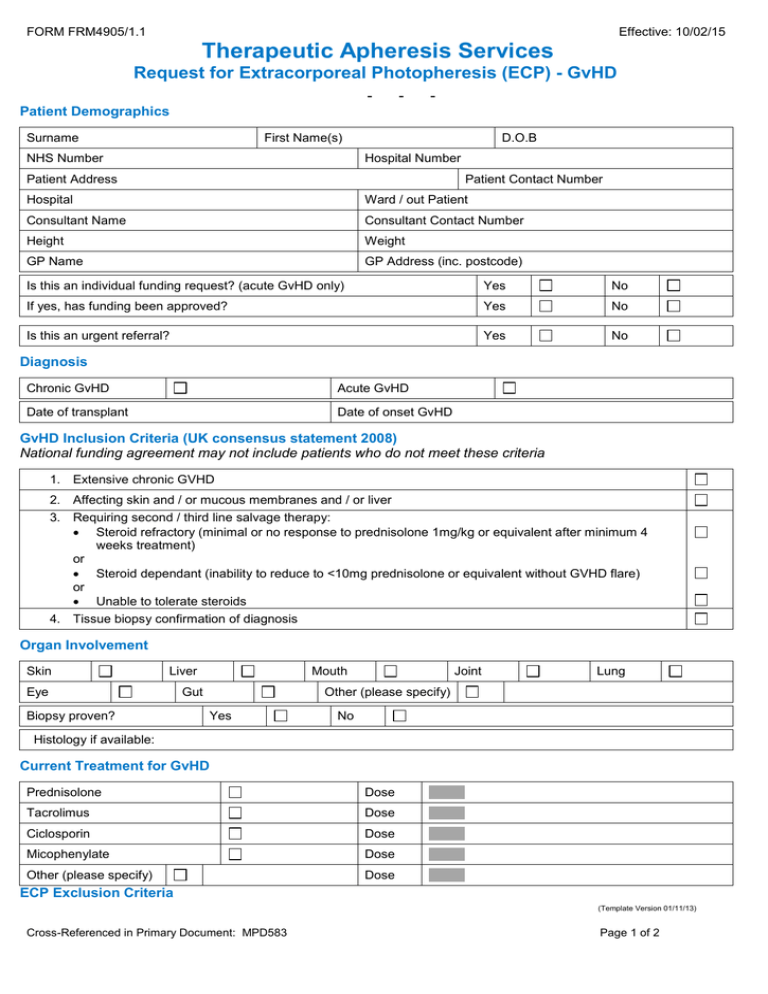
FORM FRM4905/1.1 Effective: 10/02/15 Therapeutic Apheresis Services Request for Extracorporeal Photopheresis (ECP) - GvHD - - - Patient Demographics Surname First Name(s) NHS Number D.O.B Hospital Number Patient Address Patient Contact Number Hospital Ward / out Patient Consultant Name Consultant Contact Number Height Weight GP Name GP Address (inc. postcode) Is this an individual funding request? (acute GvHD only) Yes No If yes, has funding been approved? Yes No Is this an urgent referral? Yes No Diagnosis Chronic GvHD Acute GvHD Date of transplant Date of onset GvHD GvHD Inclusion Criteria (UK consensus statement 2008) National funding agreement may not include patients who do not meet these criteria 1. Extensive chronic GVHD 2. Affecting skin and / or mucous membranes and / or liver 3. Requiring second / third line salvage therapy: Steroid refractory (minimal or no response to prednisolone 1mg/kg or equivalent after minimum 4 weeks treatment) or Steroid dependant (inability to reduce to <10mg prednisolone or equivalent without GVHD flare) or Unable to tolerate steroids 4. Tissue biopsy confirmation of diagnosis Organ Involvement Skin Liver Eye Biopsy proven? Mouth Gut Joint Lung Other (please specify) Yes No Histology if available: Current Treatment for GvHD Prednisolone Dose Tacrolimus Dose Ciclosporin Dose Micophenylate Dose Other (please specify) Dose ECP Exclusion Criteria (Template Version 01/11/13) Cross-Referenced in Primary Document: MPD583 Page 1 of 2 FORM FRM4905/1.1 Effective: 10/02/15 Therapeutic Apheresis Services Request for Extracorporeal Photopheresis (ECP) - GvHD - - - 1. Pregnancy Yes No 2. History of heparin induced thrombocytopenia Yes No 3. Uncontrolled infection Yes No 4. Photosensitivity Yes No 5. Sensitivity to psoralen compounds Yes No 6. Aphakia Yes No Yes No Yes No Yes No 7. Neutrophil count < 1 x 109 / 1 8. Platelet count < 20 x 109 /1 9. Severe diarrhoea > 1000ml daily Other Information Additional details Including previous treatment: Does the patient have any allergies? Yes No (If yes, please specify) Does the patient have any significant co-morbidities which would require modification of the procedure (e.g. cardiovascular, renal, bleeding diathesis)? Vascular Access (ECP requires 16g access in antecubital fossa and second access in opposite limb of at least 20g) Are peripheral veins adequate for apheresis? Yes No Is an apheresis central line already in place? Yes No Will an apheresis central line be inserted? Yes No (If yes, please advise date of insertion) Detail of member of staff completing this form Name: Grade: Phone Number: Date: Email address: Signature: (*To ensure confidentiality please ensure an nhs.net email address is provided) For use by NHS Blood & Transplant ONLY Has the referral been accepted? Yes No Comments: (Template Version 01/11/13) Cross-Referenced in Primary Document: MPD583 Page 2 of 2