NZQA unit standard 23676 version 2
advertisement
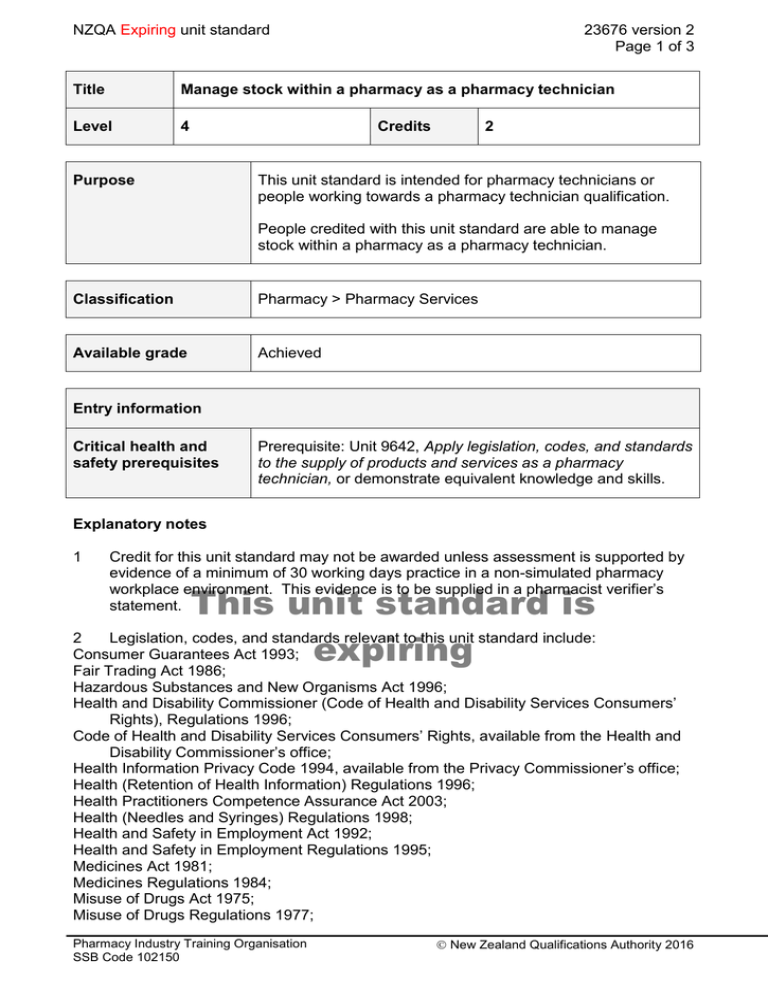
NZQA Expiring unit standard 23676 version 2 Page 1 of 3 Title Manage stock within a pharmacy as a pharmacy technician Level 4 Purpose Credits 2 This unit standard is intended for pharmacy technicians or people working towards a pharmacy technician qualification. People credited with this unit standard are able to manage stock within a pharmacy as a pharmacy technician. Classification Pharmacy > Pharmacy Services Available grade Achieved Entry information Critical health and safety prerequisites Prerequisite: Unit 9642, Apply legislation, codes, and standards to the supply of products and services as a pharmacy technician, or demonstrate equivalent knowledge and skills. Explanatory notes 1 Credit for this unit standard may not be awarded unless assessment is supported by evidence of a minimum of 30 working days practice in a non-simulated pharmacy workplace environment. This evidence is to be supplied in a pharmacist verifier’s statement. This unit standard is 2 Legislation, codes, and standards relevant to this unit standard include: Consumer Guarantees Act 1993; expiring Fair Trading Act 1986; Hazardous Substances and New Organisms Act 1996; Health and Disability Commissioner (Code of Health and Disability Services Consumers’ Rights), Regulations 1996; Code of Health and Disability Services Consumers’ Rights, available from the Health and Disability Commissioner’s office; Health Information Privacy Code 1994, available from the Privacy Commissioner’s office; Health (Retention of Health Information) Regulations 1996; Health Practitioners Competence Assurance Act 2003; Health (Needles and Syringes) Regulations 1998; Health and Safety in Employment Act 1992; Health and Safety in Employment Regulations 1995; Medicines Act 1981; Medicines Regulations 1984; Misuse of Drugs Act 1975; Misuse of Drugs Regulations 1977; Pharmacy Industry Training Organisation SSB Code 102150 New Zealand Qualifications Authority 2016 NZQA Expiring unit standard 23676 version 2 Page 2 of 3 New Zealand Code of Good Manufacturing Practice for Manufacture and Distribution of Therapeutic Goods (GMP Code), Part 4 (Wholesaling of Medicines and Medical Devices) and Part 5 (Uniform Recall Procedure for Medicines and Medical Devices), available from Medsafe; The New Zealand Pharmaceutical Schedule, available from http://www.pharmac.govt.nz; Pharmacy Council of New Zealand Code of Ethics 2004, available at http://www.pharmacycouncil.org.nz; Privacy Act 1993. Other requirements applicable to this unit standard may include but are not limited to: Pharmacy Practice Handbook and Quality Standards for Pharmacy in New Zealand, both available from the Pharmaceutical Society of New Zealand Inc. Any legislation or other requirement superseding any of the above will apply, pending review of this unit standard. 3 Definition Standard operating procedures – written documentation of the specified way to perform an activity. Outcomes and evidence requirements Outcome 1 Manage stock within a pharmacy as a pharmacy technician. Evidence requirements 1.1 Stock and associated documentation is managed in terms of batch numbers, expiry dates, ordering, storage requirements, and recall in accordance with standard operating procedures and legal requirements. storageunit requirements include but are not limitedis to – temperature, This standard cold chain, safety and security; stock – refrigerated items, controlled drugs, medicines. expiring Range This unit standard is expiring. Assessment against the standard must take place by the last date for assessment set out below. Status information and last date for assessment for superseded versions Process Version Date Last Date for Assessment Registration 1 23 April 2007 31 December 2017 Review 2 16 April 2015 31 December 2017 Consent and Moderation Requirements (CMR) reference 0128 This CMR can be accessed at http://www.nzqa.govt.nz/framework/search/index.do. Pharmacy Industry Training Organisation SSB Code 102150 New Zealand Qualifications Authority 2016 NZQA Expiring unit standard 23676 version 2 Page 3 of 3 Please note Providers must be granted consent to assess against standards (accredited) by NZQA, before they can report credits from assessment against unit standards or deliver courses of study leading to that assessment. Industry Training Organisations must be granted consent to assess against standards by NZQA before they can register credits from assessment against unit standards. Providers and Industry Training Organisations, which have been granted consent and which are assessing against unit standards must engage with the moderation system that applies to those standards. Requirements for consent to assess and an outline of the moderation system that applies to this standard are outlined in the Consent and Moderation Requirements (CMR). The CMR also includes useful information about special requirements for organisations wishing to develop education and training programmes, such as minimum qualifications for tutors and assessors, and special resource requirements. This unit standard is expiring Pharmacy Industry Training Organisation SSB Code 102150 New Zealand Qualifications Authority 2016



