12596016_Main.doc (502.5Kb)
advertisement

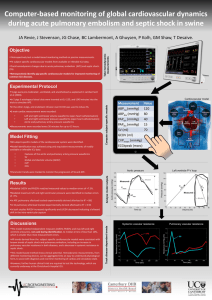
Diagnosis Using a Minimal Cardiac Model Including Reflex Actions C.E.Hann1, J.G. Chase1, S. Andreassen2, B. W. Smith2, G. M. Shaw3 1Department of Mechanical Engineering, University of Canterbury, Christchurch, New Zealand for Model Based Medical Decision Support, Aalborg University, Aalborg, Denmark 3Department of Intensive Care Medicine, Christchurch Hospital, Christchurch, New Zealand 2Centre Objectives Simulate two common disease states from onset including reflex actions Develop a linear and convex identification method that only requires data readily available in an intensive care unit (ICU) Accurately identify each time varying disease state as well as all other patient parameters in the presence of 10% uniformly distributed noise. Methodology A minimal cardio-vascular system (CVS) model [1] is employed. Reflex actions include: vaso-constriction, venous constriction, increased heart rate and increased ventricular contractility, as incorporated every heartbeat. Integral-based parameter identification [2,3] is used to identify model parameters in each 8-beat period. The pressure waveforms through the aorta and pulmonary artery, cardiac output and the maximum and minimum ventricular volumes with 10% uniform noise are the assumed measurements. Pulmonary Embolism Pulmonary Artery Pressure (mmHg) Introduction Heart disease is difficult to diagnose due to often confusing clinical a data. A minimal cardiac model has been developed that captures the major dynamics of the cardiovascular system (CVS). To assist medical staff in diagnosis and treatment, a fast accurate patient-specific parameter identification method, which can account for time varying disease state and the body’s natural reflex response, is required. Rpul increases 20% every 8 beats to simulate pulmonary embolism Mean arterial pressure: slightly down - Cardiac output: down Pulmonary vein pressure: substantially increased The integral-based identification method identified each disease state within 9% in the presence of 10% uniformly distributed noise, as shown in Tables 1 and 2. All other parameters, including blood inertances, are identified with a total mean error of 7.6%. Without including inertances (brackets), total mean error was 4.3%. Results Pericardial Tamponade Aortic Pressure (mmHg) V0,pcd decreases 20 ml every 8 heart beats for 40 beats simulating pericardial tamponade TABLE 1: PERICARDIAL TAMPONADE (DETERMINING V0,pcd (ml)) Change True value Optimized value (ml) Error (%) V0,pcd Mean (all parameters) Standard Deviation First 180 176 2.22 5.60 (2.32) 8.89 (3.23) Second 160 158 1.25 8.03 (4.45) 9.32 (4.41) Third 140 138 1.43 6.84 (2.96) 9.29 (5.18) Fourth 120 117 2.50 8.95 (5.13) 10.46 (4.15) Fifth 100 100 0 8.58 (5.22) 10.17 (5.37) TABLE 2: PULMONARY EMBOLISM (DETERMINING Rpul (mmHg s ml-1)) Mean arterial pressure: 100 to 88 mmHg - Cardiac output: 5.3 to 4.1 L/min – Pulmonary vein pressure: 2 to 7.9 mmHg. References [1] B.W. Smith, J.G. Chase, R.I. Nokes, G.M. Shaw and G. Wake. Minimal haemodynamic system model including ventricular interaction and valve dynamics. Med. Eng. Phys, 26(2):131-139,2004. [2] C.E. Hann, J.G. Chase, G.M. Shaw, and B.W. Smith. Identification of patient specific parameters for a minimal cardiac model. Proc 26th International Conf of IEEE Engineering in Med and Biology Society (EMBS 2004), San Francisco, CA, Sept 1-5, pages 813-816,2004. [3] C.E. Hann, J.G. Chase, J. Lin, T. Lotz, C.V. Doran, and G.M. Shaw. Integral-based parameter identification for longterm dynamic verification of a glucose-insulin system model. Computer Methods and Programs in Biomedicine, 77(3):259-270, 2005. Change True value Optimized value Error (%) Rpul Mean (all parameters) Standard Deviation First 0.1862 0.1907 2.41 7.93 (3.36) 10.01 (6.35) Second 0.2173 0.2050 5.67 6.32 (2.92) 7.62 (3.94) Third 0.2483 0.2694 8.50 7.26 (4.55) 9.50 (5.46) Fourth 0.2794 0.2721 2.60 8.90 (6.71) 10.84 (5.37) Fifth 0.3104 0.2962 4.59 7.64 (4.89) 9.61 (5.24) Conclusion The model accurately captured the physiological trends in two common disease states simulated from onset. Integral based optimization successively identified each disease state in the presence of significant measurement noise. These results demonstrate the potential of using this model in a clinical setting.