Test2Objectives.docx
advertisement
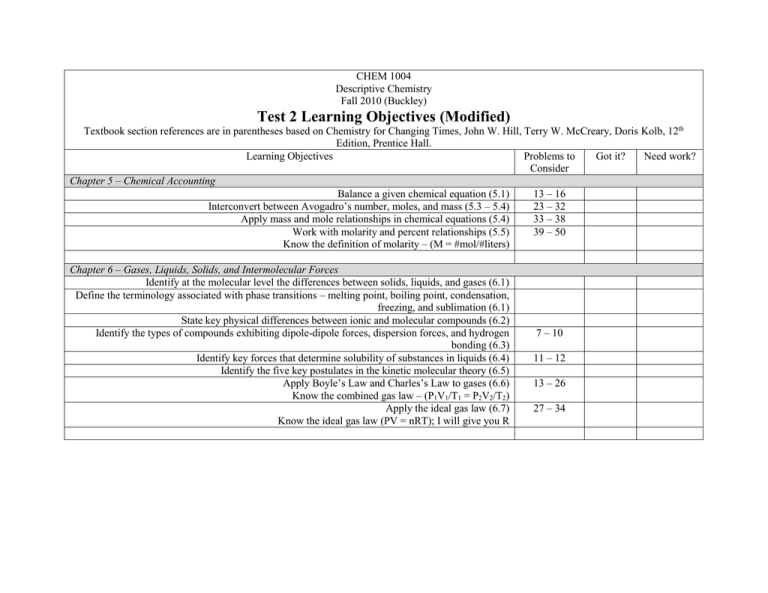
CHEM 1004 Descriptive Chemistry Fall 2010 (Buckley) Test 2 Learning Objectives (Modified) Textbook section references are in parentheses based on Chemistry for Changing Times, John W. Hill, Terry W. McCreary, Doris Kolb, 12th Edition, Prentice Hall. Learning Objectives Problems to Got it? Need work? Consider Chapter 5 – Chemical Accounting Balance a given chemical equation (5.1) 13 – 16 Interconvert between Avogadro’s number, moles, and mass (5.3 – 5.4) 23 – 32 Apply mass and mole relationships in chemical equations (5.4) 33 – 38 Work with molarity and percent relationships (5.5) 39 – 50 Know the definition of molarity – (M = #mol/#liters) Chapter 6 – Gases, Liquids, Solids, and Intermolecular Forces Identify at the molecular level the differences between solids, liquids, and gases (6.1) Define the terminology associated with phase transitions – melting point, boiling point, condensation, freezing, and sublimation (6.1) State key physical differences between ionic and molecular compounds (6.2) Identify the types of compounds exhibiting dipole-dipole forces, dispersion forces, and hydrogen bonding (6.3) Identify key forces that determine solubility of substances in liquids (6.4) Identify the five key postulates in the kinetic molecular theory (6.5) Apply Boyle’s Law and Charles’s Law to gases (6.6) Know the combined gas law – (P1V1/T1 = P2V2/T2) Apply the ideal gas law (6.7) Know the ideal gas law (PV = nRT); I will give you R 7 – 10 11 – 12 13 – 26 27 – 34