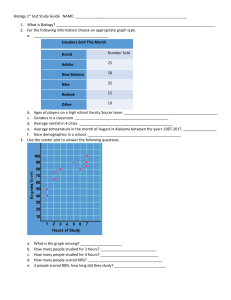
Quiz3Objectives.docx
advertisement
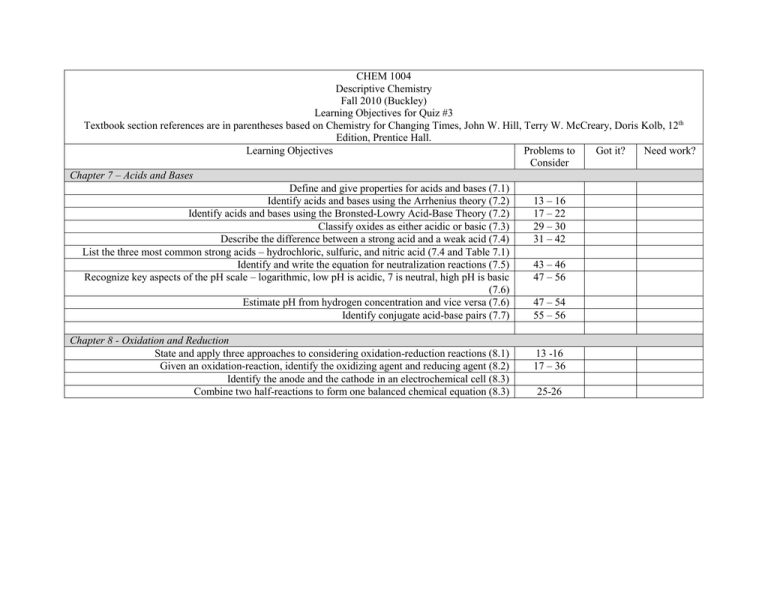
CHEM 1004 Descriptive Chemistry Fall 2010 (Buckley) Learning Objectives for Quiz #3 Textbook section references are in parentheses based on Chemistry for Changing Times, John W. Hill, Terry W. McCreary, Doris Kolb, 12th Edition, Prentice Hall. Learning Objectives Problems to Got it? Need work? Consider Chapter 7 – Acids and Bases Define and give properties for acids and bases (7.1) Identify acids and bases using the Arrhenius theory (7.2) 13 – 16 Identify acids and bases using the Bronsted-Lowry Acid-Base Theory (7.2) 17 – 22 Classify oxides as either acidic or basic (7.3) 29 – 30 Describe the difference between a strong acid and a weak acid (7.4) 31 – 42 List the three most common strong acids – hydrochloric, sulfuric, and nitric acid (7.4 and Table 7.1) Identify and write the equation for neutralization reactions (7.5) 43 – 46 Recognize key aspects of the pH scale – logarithmic, low pH is acidic, 7 is neutral, high pH is basic 47 – 56 (7.6) Estimate pH from hydrogen concentration and vice versa (7.6) 47 – 54 Identify conjugate acid-base pairs (7.7) 55 – 56 Chapter 8 - Oxidation and Reduction State and apply three approaches to considering oxidation-reduction reactions (8.1) Given an oxidation-reaction, identify the oxidizing agent and reducing agent (8.2) Identify the anode and the cathode in an electrochemical cell (8.3) Combine two half-reactions to form one balanced chemical equation (8.3) 13 -16 17 – 36 25-26