Quiz2.docx
advertisement
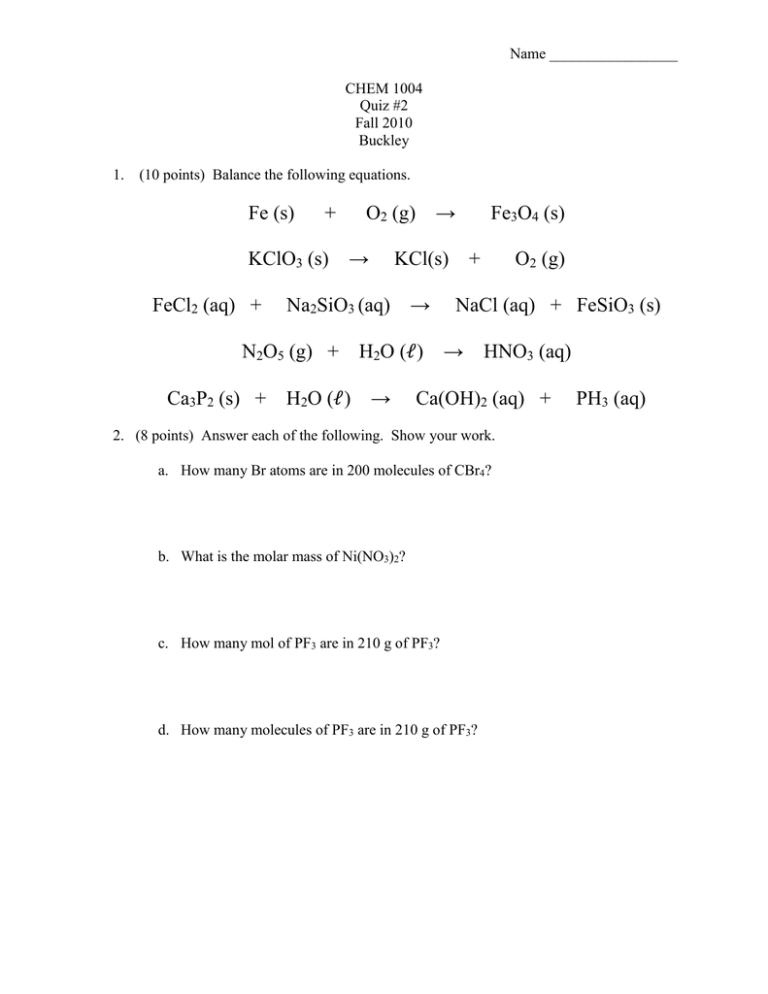
Name _________________ CHEM 1004 Quiz #2 Fall 2010 Buckley 1. (10 points) Balance the following equations. Fe (s) + KClO3 (s) FeCl2 (aq) + Ca3P2 (s) + → KCl(s) Na2SiO3 (aq) N2O5 (g) + → O2 (g) → H2O (ℓ) H2O (ℓ) → Fe3O4 (s) + O2 (g) NaCl (aq) + FeSiO3 (s) → HNO3 (aq) Ca(OH)2 (aq) + 2. (8 points) Answer each of the following. Show your work. a. How many Br atoms are in 200 molecules of CBr4? b. What is the molar mass of Ni(NO3)2? c. How many mol of PF3 are in 210 g of PF3? d. How many molecules of PF3 are in 210 g of PF3? PH3 (aq) 3. (5 points) Consider the combustion of octane (similar to gasoline): 2 C8H18 (ℓ) + 25 O2 (g) → 16 CO2 (g) + 18 H2O (g) Suppose 100 g of C8H18 is burned. a. How many mol of C8H18 are in 100 g of C8H18? b. How many mol of CO2 could be formed? c. What mass of CO2 could be formed? 4. (6 points) First draw the Lewis structure for the following compounds. Then identify the intermolecular forces – dispersion, dipole-dipole, and/or H-bonding - that would exist between the following molecules. (I gave you the last Lewis structure.) Compound Lewis structure CHF3 H2S CH3OH H | _ H-C–O–H | ─ H Types of intermolecular forces







