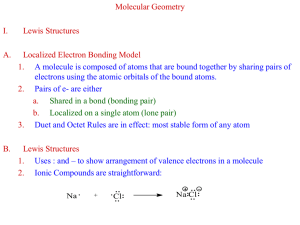
LewisstructureCO32-.docx
advertisement
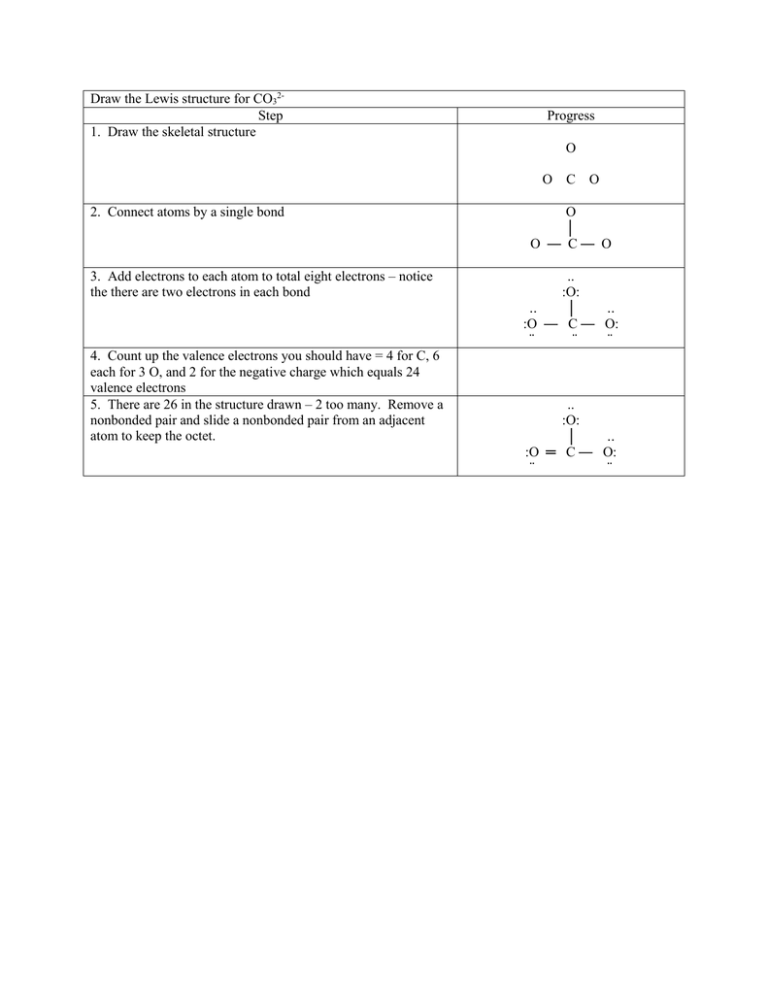
Draw the Lewis structure for CO32Step 1. Draw the skeletal structure Progress O O C O 2. Connect atoms by a single bond 3. Add electrons to each atom to total eight electrons – notice the there are two electrons in each bond 4. Count up the valence electrons you should have = 4 for C, 6 each for 3 O, and 2 for the negative charge which equals 24 valence electrons 5. There are 26 in the structure drawn – 2 too many. Remove a nonbonded pair and slide a nonbonded pair from an adjacent atom to keep the octet. O │ O — C— O .. :O: .. │ .. :O — C — O: ¨ ¨ ¨ .. :O: │ .. :O ═ C — O: ¨ ¨