Modification Request Form
advertisement

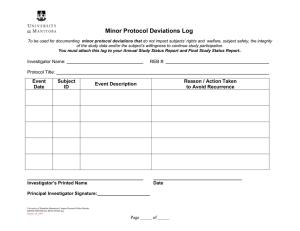
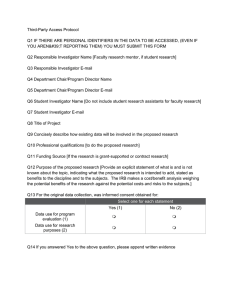
Institutional Review Board FWA00003873 Request for Modification of Human Subjects Research Protocol Protocol Information Investigator(s): Project Title: Protocol #: Modification Description Will there be a change of Principal Investigator or a change in or addition to other project staff? No Yes (please attach CITI training completion reports for new personnel) If yes, please explain and list new personnel and their role in the study: Are there planned changes in your research activities? No Yes If yes, please explain: Will your Informed Consent form be revised? No Yes If yes, please attach a copy of the new form. Submit forms via email to leah.vargas@csus.edu. Please copy your faculty sponsor if you are a student investigator. Your electronic submission of this form certifies that the planned changes in the study will not adversely affect the human subjects and that the use of human subjects is in accordance with federal and University regulations. Any additional proposed modifications in the research activities must be presented to the IRB for approval prior to implementation.