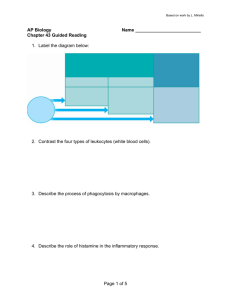
R RA
advertisement

R Reporter Antigen Popliteal Lymph Node Assay recognition of “infectious non self” and initiation of events that induce adaptive immune responses. Rather, special emphasis will be placed here on the unique humoral immune characteristics of the rabbit system. The cells and molecules that contribute to cell-mediated immunity, though less well characterized than those of mice and humans, appear to be comparable. 3 RA 3 3 RA-PLNA Reporter Antigen Popliteal Lymph Node Assay 3 Rabbit Characteristics An extensive review of the rabbit immune system has been published previously (1). Many key ideas about the immune system were first developed through studies of the rabbit model. The rabbit is rich in genetic variants ( allotypes) that provided markers used for documenting allelic exclusion, cis expression of linked genes, and germline recombination within the heavy chain locus. Figure 1 is a stick diagram of a rabbit immunoglobulin G molecule, showing some structural features and the locations of just a few of the many markers that distinguish inheritable sequence differences of heavy and light chains. Although a normal IgG molecule would have two identical light chains, in this illustration the upper light chain depicts an unusual inter-domain disulfide bond that connects the variable and constant domains of most kappa chains of Cκ1 type (allotypes b4, b5, b6, and some light chains from b9 type). The lower 3 Rabbit Immune System 3 Rabbit Immune System Rose G Mage Molecular Immunogenetics Section Laboratory of Immunology NIAID Building 10 11 N 311, MSC 1892 NIH, 10 Center Drive Bethesda, MD USA Synonyms Rabbit, Oryctolagus cuniculus. Definition The rabbit immune system consists of the organs, tissues, cells, and molecules that interact to contribute to specific responses to foreign antigens, infectious agents, or—in autoimmune conditions—to self antigens. Included among the important molecules are the genes and gene products that are necessary for the development and proper functioning of the immune system including antigen-specific T cell and B cell receptors and B cell-secreted immunoglobulins. The innate immune system that constitutes the first line of defense will not be considered here, although it is now clearly recognized as important for early Rabbit Immune System. Figure 1 Stick diagram of rabbit immunoglobulin G showing locations of disulfide bonds and some allelic types (allotypes) of heavy and light chains. 546 Rabbit Immune System light chain is drawn with only the intra-domain disulfide bonds typically found in other species, as well as in rabbit kappa 2 and lambda light chains. I have speculated that the great stability and long “shelf life” of rabbit antibodies may in part result from stabilization of kappa 1 light chain structures by the unusual inter-domain disulfide bond. Conversely, the Cys at position 80 in most rabbit Vκ genes presents a problem in generating recombinant chimeric Fab molecules with human Cκ because the Cys 80 in Vκ leads to an unpaired thiol group. Compared to b4 rabbits, those of the rare b9 and mutant bas types express a higher proportion of Vκ that lack the Cys 80. When rabbits of these types were immunized and recombinant rabbit-human Fab generated by phage display, yields of distinct and specific high affinity Fab increased (2). A summary of some of the rabbit kappa and lambda genetic types and the organization of the kappa and lambda light chain loci is shown in Figure 2. The rabbit has an unusual duplication of the kappa light chain locus (Cκ1 and Cκ2 in Figure 2). The allelic forms of the Cκ1 genes b4, b5, b6 and b9 differ by multiple amino acids in their constant regions and seem to have somewhat different sets of associated Vκ genes. There are more than 100 different Vκ genes but they are not fully mapped and sequenced. Some of Vκ genes and Cκ2 are located about 2 Mb away from Cκ1 in the duplicated rabbit kappa locus. In wild-type rabbits, kappa 1 light chains are the major expressed isotype along with 10–30% of lambda light chains. However, in the mutant Basilea strain (bas) which has a defective acceptor site for splicing Jκ to Cκ in mRNA for kappa 1 light chains, there is elevated expression of both kappa 2 and lambda light chains. The allelic forms of kappa 2 chains are the result of a single amino acid replacement change in the Cκ2 sequences. Figure 3 shows a schematic diagram of the heavy chain locus with VH (the first few of more than 100 VH genes are shown), DH and JH genes, and the genes that encode the constant regions of IgM, IgG, IgE and IgA (μ, γ, ε and α). Rabbits are again unusual in having only one γ gene but 13 α genes. A gene encoding a rabbit homologue of IgD has not been identified in the region downstream of rabbit μ where the δ gene is found in some species. Perhaps most unusual of all are the inherited forms of heavy chain variable regions that are detectable using anti-allotype antisera raised by immunization of rabbits of one type with IgG of another type. The reason why allelic forms of rabbit heavy chain variable regions are detectable became clear when it was found that in most rabbit B lymphocytes, the first gene in the locus, VH1 is rearranged; the different allelic forms have amino acid differences encoded by the VH1 alleles in framework regions 1 and 3. This VH1 gene can rearrange to one of several DH genes and one of three functional JH genes, to form VHDHJH. As shown in Figure 3, VH1 is usually rearranged. In a mutant strain named Alicia (ali), the VH1a2 gene is deleted and the first gene that is functional, VH4, is frequently found rearranged along with a few other upstream genes. Rabbit Immune System. Figure 2 Diagram (not to scale) of the rabbit light chain kappa and lambda loci. Rabbit Immune System. Figure 3 Diagram (not to scale) of the rabbit heavy chain locus. Rabbit H- and L-Chain Diversity is Generated by Rearrangements, Somatic Hypermutation and Gene Conversion Today the rabbit remains a major source of polyclonal antibodies found in catalogs of commercial suppliers. There are some unique characteristics of the immune system of rabbits that contribute to their special capability to produce diverse highly specific high-affinity polyclonal antibodies. These include use of both gene conversion (GC) and somatic hypermutation (SHM) to alter the sequences of rearranged antibody heavy and light chain genes; selection of favorable amino acid replacements during clonal expansion of antigen-specific B lymphocytes in germinal centers; great diver- 3 3 Rabbit Immune System sity of kappa light chain variable region genes; and unusual germline Vκ-encoded variability of the length of complementarity-determining region 3 (LCDR3). Compensation for limited heavy chain VHDHJH by diverse light chain VκJκ occurs even before the start of somatic diversification processes. Despite this, gene conversion further diversifies rearranged VκJκ both in the appendix of young non-immunized rabbits and in the spleens and lymph nodes of immunized rabbits. The rabbit VHa allotypes are encoded by the 3’ VH1 gene which rearranges in most B cells. Some diversity is generated by the choice of one of several DH and JH genes. Even before diversification by GC and SHM, there is diversity generated at the sites of VH to DH and DH to JH DNA recombination by insertions and deletions of bases at the sites of joining. At the points of joining, the additions and deletions of bases that occur lead to great variability in the sequences of the heavy chain third complementarity determining region (HCDR3). In most rabbit B cells, only one chromosome of the allelic pair undergoes complete rearrangement, and the order of arrangement may also be VH to DH followed by VHDH to JH; this differs from the order DH to JH followed by VH to DHJH shown in most textbooks. The sequence of the rearranged VHDHJH gene is further diversified by gene-conversion-like changes. Sequence blocks that vary in length are acquired from upstream donor VH genes. This was first described as the mechanism of VH-gene diversification in the chicken, where it occurs in specialized gut-associated lymphoid tissue (GALT), the bursa of Fabricius of embryos and young chicks, and later in life in splenic germinal centers. In young rabbits, these changes also take place in specialized GALT sites such as the appendix (3,4), and in older rabbits in germinal centers of spleens and lymph nodes in response to foreign antigens. Comparisons of the chicken bursa and rabbit appendix were first published in the 1960s and suggested that the rabbit appendix might be a homologue of the chicken bursa, based on similarities in follicle development and the finding that neonatal thymectomy of rabbits had no effect on appendix development. The independence of appendix cell development from the thymus, as well as remarkable histological resemblance, suggested that rabbit appendix may be a central lymphoid tissue analogous to chicken bursa. Subsequently it was shown that removal of appendix and Peyer’s patches resulted in severe depletion of B cells and blunted immune responses. Once gene conversion was discovered to contribute to sequence diversification in both chicken bursa and rabbit appendix, it was also shown that removal of rabbit GALT structures limited—but did not eliminate—diversification of rearranged heavy chain sequences. There are similarities and differences between development and diversification of B cells in 547 the two species, some of which are summarized in Table 1. Rabbit Central and Peripheral B cell Development and V Gene Diversification In rabbit appendix, development of the primary preimmune antibody repertoire requires endogenous gut flora. The gut flora may primarily provide B cell survival and proliferation signals, either directly or indirectly through interactions that activate the innate immune system. The rearranged VHDHJH and VLJL in appendix B cells diversify by gene conversion and somatic hypermutation but the receptors may not be specific for a provoking antigen (4). The sequences of rearranged heavy and light chains within a single expanding clone are strikingly diverse in CDR3. This led to the view that cells that diversify within individual clones in appendix may not develop receptors specific for a single antigenic epitope; the clonal diversification contrasts with the response to specific antigens in peripheral lymphoid tissues such as the spleen, lymph nodes, and Peyer’s patches where germinal centers develop. There, B cells also diversify rearranged heavy and light chain sequences by somatic hypermutation and gene conversion. This antigen-driven diversification leads to increased affinity of the receptors on some B cells. Selection for cells with good affinity for the immunizing antigen occurs via interactions with antigen on the surface of specialized follicular dendritic cells (FDC). The cells with high affinity may process antigen picked up from FDC and present processed antigen to germinal center T cells which then release stimuli for proliferation, class switching and development into plasma cells or memory B cells. Gene conversion and somatic mutation may also decrease the affinity of antigen receptors. Cells with decreased affinity may die by apoptosis or possibly undergo further rounds of mutation and selection. We have also speculated that in adults peripheral germinal centers may have a secondary role comparable to the role of appendix in young rabbits. For example if some cells with decreased affinity survive and exit as antigen-responsive cells, the germinal centers could be a source of new repertoire in adults. Rabbit Leukocyte Markers, Chemokines and Cytokines Tables of rabbit leukocyte antigens, T cell receptors and associated proteins and accessory molecules involved in signaling, leukocyte and endothelial adhesion molecules and some chemotactic molecules described in rabbits for which probes and/or monoclonal antibodies are available can be found in reference (1). Data on cytokines and chemokines summarized at the time of this publication were limited. Although some progress has been made in this area (5), no commercial kits are available for rabbit. Some reagents specific for R 548 Rabbit Immune System Rabbit Immune System. Table 1 Similarities and differences between chicken bursa and rabbit appendix B cell development Chicken Bursa Rabbit Appendix VDJ and VLJL rearrangements in spleen, yolk sac Generally, rearrangement on only one chromosome VDJ and VLJL rearrangements in bone marrow, fetal omentum, fetal liver, young spleen Generally, rearrangement on only one chromosome. Migration to embryonic bursa Migration to newborn appendix Rapid B cell expansion in bursal follicles even before exposure to exogenous (foreign) antigens Endogenous stimuli not characterized Diversification by gene conversion and SHM pre-and post-hatching to develop preimmune repertoire Rapid B cell expansion requires presence of gut flora (exogenous) There may be some effects of endogenous stimuli such as CD5 Diversification by gene conversion and somatic hypermutation (SHM) after about 2 weeks of age to develop preimmune repertoire Emigration from bursa to periphery Bursa involutes by sexual maturity Emigration from appendix to periphery Appendix changes in appearance and possibly function but does not involute Emigrants represent the chicken’s preimmune repertoire Further diversification by gene conversion and hypermutation occurs in germinal centers of spleen after immunization Emigrants thought to represent rabbit’s preimmune repertoire However, the diversification seen in spleen after immunization suggests some new B cells may also seed adult spleen and initiate germinal centers human markers (produced in species other than rabbit) cross-react with homologous rabbit proteins. Preclinical Relevance The special characteristics of the rabbit immune system that lead to high affinity and specificity of antibodies described above make the rabbit a major source of polyclonal antibodies used in diagnostics and immunopathology. Although rabbits have been used in toxicology for tests of eye irritation potential (Draize rabbit eye irritancy test), as well as for tests of dermal toxicity, many members of the scientific community and animal welfare organizations have criticized the tests as subjective and inhumane. In the United States, the validation status of in vitro screening assays for ocular irritation is currently being evaluated. stat.com), is widely used for the treatment of renal transplant acute rejection, in conjunction with concomitant immunosuppression. However, such a therapeutic cannot be used to treat patients who are not immunosuppressed because they would mount immune responses to the foreign rabbit immunoglobulin. Attempts are currently under way to genetically engineer rabbits that will produce therapeutic human polyclonals (http://www.polyclonals.com). The technology for production of rabbit monoclonal antibodies has also developed to the point that highly specific highaffinity rabbit monoclonal antibodies may find use in drug discovery, diagnostics and possibly as the starting point for development of humanized therapeutic monoclonals (http://www.epitomics.com/technology/ tech.html). Relevance to Humans References Rabbit models for diseases of immunological relevance include various infectious diseases such as anthrax, syphilis, tuberculosis, virus-induced papilloma, and HTLV1: a rabbit model of hemolytic disease of newborns; complement deficiencies; and a variety of autoimmune diseases. Rabbits have been used as the starting source of potential humanized therapeutic monoclonal antibodies because they produce highly specific antibodies with high affinities (2). A polyclonal rabbit anti-human thymocyte globulin (Thymoglobulin), approved by the United States Food and Drug Administration in December 1998 (http://www.sang- 1. Mage RG (1998) Immunology of lagomorphs. In: Pastoret PP, Bazin H, Griebel HP, Govaerts H (eds) Handbook of Vertebrate Immunology. Academic Press, London, pp 223–260 2. Popkov M, Mage RG, Alexander CB, Thundivalappil S, Barbas CF, Rader C (2003) Rabbit immune repertoires as sources for therapeutic monoclonal antibodies: the impact of kappa allotype-correlated variation in cysteine content on antibody libraries selected by phage display. J Molec Biol 325:325–335 3. Pospisil R, Mage RG (1998) Rabbit appendix: A site of development and selection of the B cell repertoire. In: Kelsoe G, Flajnik M (eds) Current Topics in Microbiology and Immunology ,Vol 229: Somatic Diversification Real-Time Polymerase Chain Reaction Ras Ras is a small-molecular-weight G protein responsible for regulating the MAP kinase cascades, which lead to activation of transcription factors. Signal Transduction During Lymphocyte Activation 3 of Immune Responses. Springer-Verlag, Heidelberg, pp 59–70 4. Seghal D, Obiakor H, Mage RG (2002) Distinct clonal Ig diversification patterns in young appendix compared to antigen-specific splenic clones. J Immunol 168:5424– 5433 5. Perkins HD, van Leeuwen BH, Hardy CM, Kerr PJ (1999) The complete cDNA sequences of IL-2, IL-4, IL-6 and IL-10 from the European rabbit (Oryctolagus cuniculus). Cytokine 12:555–565 549 Rat Immune System 3 Radiation Mucositis Rodent Immune System, Development of the Oral Mucositis and Immunotoxicology 3 Reaction Radioimmunoassay (RIA) 3 An immunoassay that is based on the use of radioactivity (e.g. 125Iodine-labeled antigens) to generate counts per minute upon the binding of a radiolabeled antigen with its antibody. Immunoassays Delayed-Type Hypersensitivity Reactive Oxygen Intermediate (ROI) 3 3 An experimental design utilizing several homogeneous groups of subjects. There are as many subjects in a block as there are treatment conditions, and within each block subjects are randomly assigned to treatment conditions. Statistics in Immunotoxicology 3 Randomized Complete Blocks Design Products, like hydrogen peroxide and superoxide anion, of the oxidative burst that occurs in neutrophils, macrophages, and other cells in response to phagocytosis or other forms of receptor stimulation. These reactive intermediates can be released into the phagosome, where they can attack ingested microbes, or are secreted outside the cell where they might attack extracellular pathogens, or contribute to inflammation and local tissue damage. Opsonization and Phagocytosis Antibody-Dependent Cellular Cytotoxicity 3 R Polymerase Chain Reaction (PCR) Real-Time Polymerase Chain Reaction A system that detects and quantifies gene expression or concentration of a pathogen. PCR product is monitored cycle-by-cycle by combining thermal cycling, fluorescence detection, and application-specific software. Polymerase Chain Reaction (PCR) 3 RANTES (regulated on activation normal T cell expressed and secreted; CCL5) is a member of the C-C subgroup of chemokines. RANTES is secreted by circulating T cells and is chemotactic for T cells, eosinophils, and basophils and plays an active role in recruiting leukocytes into inflammatory sites. It increases the adherence of monocytes to endothelial cells, selectively supports the migration of leukocytes, and causes the release of histamines. RANTES binds to CCR5 which is an HIV co-receptor. Cancer and the Immune System Chemokines Interferon-γ Real-Time and Quantitative PCR 3 RANTES 3 3 3 Real-Time Reverse Transcription PCR Polymerase Chain Reaction (PCR) 3 Rearrangement During B cell development in the bone marrow a rearrangement of the genomic DNA takes place. The gene encoding the variable domain of the light chain is generated by the stepwise recombination of two gene elements, the VL gene and the JL gene. The gene encoding the variable domain of the heavy chain is generated by the stepwise recombination of three gene elements, the VH gene, the DH element and the JH gene. B Cell Maturation and Immunological Memory Recombinant Transgenic Animals Recombinant Antibodies Antibody molecules produced in prokaryotic and eukaryotic cells in culture or whole animals and plants using genetic engineering methods. Antibodies, Antigenicity of 3 Real-Time Reverse Transcription PCR 3 550 Reconstructed Human Skin/Epidermis 3 Three-Dimensional Human Skin/Epidermal Models and Organotypic Human and Murine Skin Explant Systems 3 Recall Antigens Antigens, usually of microbial origin, such as tetanus toxoid or pneumococcal antigens, to which the organism has been previously exposed to and to which the organism has developed a memory capacity. Primate Immune System (Nonhuman) and Environmental Contaminants Red Pulp Part of the spleen comprising venous sinuses filled with blood and splenic cords. Main function is phagocytosis of particulate material and removal of aged erythrocytes from blood. In some species, the red pulp is a site of hematopoiesis. Spleen 3 3 Receptor Shedding Regenerative Anemia Anemia characterized by the presence of increased reticulocyte count or increased polychromasia, indicative of adequate bone-marrow response. Antiglobulin (Coombs) Test 3 Some transmembrane cytokine receptors can be released from the surface by proteolytic cleavage through ektoproteases. Receptor shedding has two effects: it rapidly deprives the target cell of functional receptors on the cell surface and thus interrupts or terminates cytokine signaling. It also provides soluble cytokine receptors which may have agonist properties, e.g. by protecting the circulating cytokine from proteolytic degradation, or may have antagonistic effects by scavenging and neutralizing cytokines. Cytokine Receptors 3 Regression Analysis Cytokine Receptors A statistical technique in which the relationship between the dependent variable and an independent variable or variables is fit using linear or nonlinear equations. Often used for deriving a prediction equation. Statistics in Immunotoxicology 3 Receptors for Mediators of the Immune System 3 Regulatory Guidance in Immunotoxicology Regulated on Activation, T Cell Expressed and Secreted (RANTES) RANTES is a chemokine involved in intracellular signaling including stimulation of G protein-coupled receptor activity, and tyrosine phosphorylation of multiple proteins. Interleukin-1β (IL-1β) 3 Regulatory Cells Specialized populations cells that modulate the function of other immune cells to prevent uncontrolled or prolonged responses. Autoimmunity, Autoimmune Diseases 3 Regulatory Environment Regulatory Guidance in Immunotoxicology 3 Regulatory Guidance in Immunotoxicology Robert V House DynPort Vaccine Company LLC 64 Thomas Johnson Drive Frederick, MD 21702 USA Synonyms Regulatory environment, guidelines in immunotoxicology. Definition From its inception in the late 1970s, immunotoxicology has developed from an essentially academic discipline to an important tool for assessing the risk of human exposure to various xenobiotics. From its early days, immunotoxicology has been virtually synonymous with immunosuppression; this is perhaps due to the dual influences of early assays used to assess immunotoxicity and the more immediately obvious sequelae of decreased host resistance in comparison to, for example, autoimmunity. However, it is increasingly recognized that any perturbation of the immune response from its tightly regulated normal range can have serious adverse consequences on health. In recognition of this, most of the regulatory guidance spe- 551 cific for immunotoxicology emphasizes individual evaluation of an agent based on prior information and its expected/intended molecular mechanism of action. In this review regulatory guidance is divided into generalized chemical class, with the understanding that overlap is inevitable. Characteristics Industrial and Environmental Chemicals Some of the earliest codified immunotoxicology test guidelines were developed to augment toxicological assessment of chemicals with some of the greatest potential for large-scale human exposure, namely pesticides. In 1996 the Office of Prevention, Pesticides and Toxic Substances (OPPTS) of the US Environmental Protection Agency (EPA) published guidelines entitled Biochemicals Test Guidelines: OPPTS 880.3550 Immunotoxicity (1), which described the preferred study design for evaluating potential immunotoxicity in biochemical pest control agents. The panel of tests included in this guideline is exceptionally thorough, including standard toxicology tests as well as many of the standard functional tests being employed at that time, including both humoral and cell-mediated immune function (the exceptions being primarily cytokine quantification and flow cytometry). Although this document explains the “how” of testing, it is lean on the “why”. To address this deficiency, a second document was published concurrently, entitled Biochemicals Test Guidelines: OPPTS 880.3800 Immune Response (2). This companion document provides a good rationale for why pesticides must be tested for immunotoxicity, together with more detailed explanations for testing strategies, and additional details on advanced (mechanistic) tests including host resistance and bone marrow function. Whereas immunotoxicity evaluation encompassed by the 880 series of guidelines would arguably detect any type of immunotoxicity, its breadth would probably render it tremendously expensive and time consuming. In 1998, the EPA followed up with Health Effects Test Guidelines: OPPTS 870.7800 Immunotoxicity (3) which described immunotoxicology testing for nonbiochemical agents that would be regulated by EPA. This document provides descriptions of both why and how, with a far more abbreviated panel of testing to be performed. While the 880 series of immunotoxicology guidelines are probably excessive, the testing approach mandated by 870.7800 has stood up well in intervening years and reflects the more limited, case-by-case approach currently favored. Most notably, the functional assessment is pared down to T-dependent antibody formation (plaque assay), natural killer (NK) cell function, and quantitation of T cells and B cells; this combination is derived from the early work of Luster and colleagues which demonstrates the greatest pre- R 552 Regulatory Guidance in Immunotoxicology dictivity of known immunotoxicants using these three assays. This study design described in this document is amenable for testing a wide range of industrial and environmental chemicals. In Europe, the Organisation for European Cooperation and Development (OECD) regulates testing of chemicals for toxicity. The OECD Guideline 407 entitled Repeated Dose 28-day Oral Toxicity Study in Rodents (4),while not specific for immunotoxicology, includes a variety of toxicological endpoints that can provide early evidence of immune system alterations. Missing, however, are any functional assays to directly measure any immune deficit. Although meetings have been held to suggest the addition of functional assays (e.g. Immunology Work Group Meeting, 11–12 December 1996), at present the 407 guideline does not include such assays. Food Additives After industrial and environmental chemicals, food additives may have the greatest potential for human exposure. In the USA these chemicals are regulated by the Food and Drug Administration’s (FDA) Center for Food Safety and Applied Nutrition. In March 1993 the FDA published the Draft Redbook II, which recommended safety testing practices for food additives. This document contained an extensive description of immunotoxicology testing; although Redbook II was never finalized, the approach was described in some detail in a number of publications (5,6). In general, the Redbook guidelines resembled the “tier” approach that was used with such success in the early development and qualification studies performed under the aegis of the National Toxicology Program. However, Redbook emphasized a step-wise approach, beginning with “retrospective level I” (expanded) studies utilizing data obtained in standard toxicology testing as an initial indicator of potential immunomodulation. Subsequent stages included enhanced (expanded) level I, level II, and enhanced (expanded) level II testing designs. This approach was very much case-by-case, with each level predicated on positive findings in its predecessor. In 2001, the FDA began offering an electronic version of Redbook, entitled Toxicological Principles for the Safety of Food Ingredients (Redbook 2000) (7). As of the writing of this review, the guidelines for immunotoxicity studies exist only in outline form in Redbook 2000. Pharmaceuticals In the USA, safety testing of small molecule pharmaceuticals is the purview of the US FDA Center for Drug Evaluation and Research (FDA CDER). In October of 2002, the CDER released a long-awaited document entitled Guidance for Industry: Immunotoxicology Evaluation of Investigational New Drugs (8). This document is arguably the most comprehensive of any published guidance, describing a diversity of adverse events including immunosuppression, immunogenicity, hypersensitivity, autoimmunity, and adverse immunostimulation. The document describes each of these types of immunotoxicity (more accurately, immunomodulation) in detail, and provides not only approaches but also suggests methodology for evaluating each type. Like the document produced by the Committee for Proprietary Medicinal Products (CMPM) (as described below) the FDA CDER guidance advocates the use of information derived from standard repeatdose toxicity studies to provide early evidence of immunotoxicity, with subsequent evaluations to be rationally designed to use a minimum of animals and resources while deriving the maximum amount of information. Subsequent to the publication of the FDA CDERthe primary purpose of this particular document was to describe an overall approach to safety testing of pharmaceuticals, it was important as the first guidance document mandating specific immunotoxicology screening for pharmaceuticals. An appendix of this document describes a staged evaluation, emphasizing that information gained in standard toxicology evaluation can be useful as a primary indicator for immunotoxicity. Functional tests may be incorporated to gain additional information, first as an initial screen and then progressing to extended studies as indicated. The choice of assays to be used includes combinations of functional tests known to be predictive of immunotoxicity, as described in the early National Toxicology Program publications. As the first published document requiring immunotoxicology evaluation, CPMP/SWP/1042/99 predictably was met with a combination of resistance and confusion. Much of this was allayed in a workshop held in Noordwijk in the Netherlands in November of 2001, sponsored by the Drug Information Association (DIA). At this meeting the intent of the guideline was clarified. A summary of the workshop has been published (11). A second CPMP document that includes reference to immunotoxicity assessment is Note for Guidance on the Quality, Preclinical and Clinical Aspects of Gene Transfer Medicinal Products (CPMP/BWP/3088/99) (12) currently in draft form. This document recognizes the possibility of adverse immunological events as a consequence of gene transfer therapy, although it makes no specific recommendations for testing. Japanese regulatory agencies have been cautious in promulgating immunotoxicology guidelines. In 1999, the Japanese Pharmaceutical Manufacturers Association (JPMA) published two documents, International Trends in Immunotoxicity Studies of Medicinal Products (13) and Survey on Antigenicity and Immunotoxicity Studies of Medicinal Products (14). These Regulatory Guidance in Immunotoxicology comprehensive documents provided a survey of immunotoxicologic methods and study designs in use in Japan and elsewhere, without advocating or requiring any studies per se. At the DIA meeting in Noordwijk (11) a representative from the Japanese Pharmaceutical Manufacturers’ Associated presented an Interim Draft Guidance for Immunotoxicity Testing (15), which describes the current thinking on such testing. As of the preparation of this review, this draft guidance document has not been published and is not readily available for review. Thus, as of 2004, there are no published Japanese guidance documents specifically regulating immunotoxicology evaluation. Biologicals Biologicals (for the purposes of this review defined as therapeutics derived by biotechnology) present a unique challenge for immunotoxicity assessment for two primary reasons. First, many of these agents (e.g. cytokines and other immunomodulatory molecules) are intended to modulate therapeutically the immune response. Therefore, it can be difficult to differentiate between the agent’s efficacy and a truly adverse reaction. Second, because many of these agents are proteins or peptides, their introduction into a host often triggers an immune response directed against the molecule itself. This can lead to alterations in pharmacodynamics, or to other adverse reactions. Thus, development of appropriate guidance on testing these agents is problematic. One approach is promulgated by the International Conference on Harmonisation (ICH) in the document Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals S6 (16). This document includes sections on immunogenicity (as described above) as well as a brief mention of immunotoxicity studies. In short, the S6 document recognizes the inappropriateness of a structured tier approach, opting instead for careful design of screening studies, followed by mechanistic studies to clarify any potential evidence of immunotoxicity. Specific techniques and approaches are not described in the S6 document. Safety evaluation of biological drugs is regulated in the USA by the FDA Center for Biologics Evaluation and Research (CBER). To date, the CBER has not promulgated any written guidance on immunotoxicology. The reason for this lack of written guidance is the extreme diversity of biological therapeutics, which makes it difficult to design a standardized testing approach. Rather, the approach of the CBER to addressing potential immunotoxicology has always been case-by-case, generally following suggestions provided in the ICH S6 document. Currently there are institutional changes underway within FDA that would put therapeutic proteins now 553 regulated by CBER under the regulatory authority of CDER; therefore the CDER guidance document could apply to these products Vaccines Along with certain biologicals, vaccines present a challenge for immunotoxicological evaluation since they are specifically designed to induce an immune response—a situation deemed undesirable (or potentially so) for most of the other agents described in this review. Since methodology is well established to evaluate the desirable immunomodulation produced by vaccine, the concern of regulatory agencies is the propensity of these therapeutics to produced undesired or deleterious effects on the immune system. European regulation of vaccines is described in Note for Guidance on Preclinical Pharmacological and Toxicological Testing of Vaccines (17) by the CPMP. In this document, immunotoxicology is to be considered during toxicology testing. In particular, vaccines should be considered for their immunological effect on toxicity, such as antibody complex formation, release of cytokines, induction of hypersensitivity reactions (either directly or indirectly), and association with autoimmunity. No specifics are described for methods or approaches; rather, each vaccine is to be evaluated on a case-by-case basis. The FDA CBER is tasked with regulating vaccines in the US. One of the primary documents describing vaccine studies is Guidance for Industry for the Evaluation of Combination Vaccines for Preventable Diseases: Production, Testing and Clinical Studies (U.S. Department of Health and Human Services, 1997). Animal immunogenicity is covered in detail in the document, although immunotoxicity is not specified as an area of concern. On the other hand, the CBER’s Considerations for Reproductive Toxicity Studies for Preventive Vaccines for Infectious Disease Indications (18). Although this is intended primarily to assess effects of vaccination on reproductive function (including generalized toxicity such as fetal malformations), it acknowledges the potential immunological reactions resulting from the vaccination process to exert unintended consequences. No specific guidance is provided on methods or approaches to be used in this evaluation. Devices and Radiological Agents It has been recognized by the FDA that immunotoxicity may result not only from chemical or biological agents that dynamically interact with the physiology of humans (such as small molecule drugs or biologicals), but also from medical devices that contact the body externally (via skin or mucosa), or internally (implantable devices), or by external communication to the blood or tissue. R 554 Regulatory Guidance in Immunotoxicology Thus, FDA Center for Devices and Radiological Health published the guidance entitled Guidance for Industry and FDA Reviewers: Immunotoxicology Testing Guidance (19) in May 1999 that addresses testing for medical devices. This guidance is based on General Program Memorandum G95-1, an FDA-modified version of International Standard ISO-10993, Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing. Immunotoxicology Testing Guidance provides detailed guidance for determining when immunotoxicity testing should be performed (including a flowchart and numerous tables), but does not provide details on which methods should be employed, or for overall study design. Some additional details on the use of this guidance were published by Anderson and Langone in 1999 (20). This manuscript, similar to the guidance, provides little information on which specific assays to use. It is, however, a useful adjunct to the guidance document. American Society for Testing and Materials The American Society for Testing and Materials (ASTM) is a not-for-profit organization promoting the development of voluntary standards for materials, products, systems and services. ASTM develops documents that serve as a basis for manufacturing, procurement, and regulatory activities. Since the ASTM standards are voluntary, they are included in this review only for the sake of completeness. The two relevant documents are F1905-98 (Standard Practice for Selecting Tests for Determining the Propensity of Materials to Cause Immunotoxicity) (21) and F1906-98 (Standard Practice for Evaluation of Immune Responses in Biocompatibility Testing Using ELISA Tests, Lymphocyte Proliferation, and Cell Migration) (22). Hypersensitivity Although much attention is paid to immunosuppression (low immune response) in the majority of guidance documents, it is hypersensitivity (hyperactive immune response) that is the most common type of immunomodulation resulting from exposure to xenobiotics. Due to the acknowledged frequency of this occurrence, as well as the multiplicity of testing methods that have been developed, a complete coverage of this condition will not be included here. However, one method for assessing hypersensitivity has taken priority in assessing contact hypersensitivity, namely the murine local lymph node assay (LLNA). Detailed explanations of this assay and its use are covered in the OECD 429 guideline, entitled Skin Sensitisation: Local Lymph Node Assay (23); the US EPA document OPPTS 870.2600 Skin Sensitization (24), and the ASTM document Standard Practice for Evaluation of Delayed Contact Hypersensitivity Using the Murine Local Lymph Node Assay (LLNA) (25). Regulatory Environment The extended bibliography below includes guidelines and guideline drafts which are to be considered for the special aspects of immunotoxicologic screenings mentioned here. Acknowledgement This article was prepared under the Immunotoxicology Workgroup supported by the EPA Office of Research and Development (National Center for Environmental Assessment), the EPA Office of Children’s Health Protection, National Institute of Environmental Health Sciences (National Toxicology Program) and National Institute for Occupational Safety and Health (Health Effects Laboratory Division). Members of the workgroup not included as authors are Laura Blanciforti (NIOSH), David Chen (EPA/OCPH), Dori Germolec (NIEHS, NTP), Michael Kashon (NIOSH), Marquea King (EPA/ORD/NCEA), Robert Luebke (EPA/ORD/ HERL) Michael Luster (NIOSH) Christine Parks (NIEHS) and Yung Yang (EPA, OPPTS). Special thanks to Bob Sonawane (EPA/ORD/NCEA) for helping to organize this effort. References 1. Biochemicals Test Guidelines: OPPTS 880.3550 Immunotoxicity. United States Environmental Protection Agency, February 1996 2. Biochemicals Test Guidelines: OPPTS 880.3800 Immune Response. United States Environmental Protection Agency, February 1996 3. Health Effects Test Guidelines: OPPTS 870.7800 Immunotoxicity. United States Environmental Protection Agency, August 1998 4. OECD Guideline for the Testing of Chemicals 407: Repeated Dose 28-day Oral Toxicity Study in Rodents. Adopted 27 July 1995 5. Hinton DM (1995) Immunotoxicity testing applied to direct food and colour additives: US FDA ‘Redbook II” Guidelines. Hum Exp Toxicol 14:143–145 6. Hinton DM (2000) US FDA “Redbook II” immunotoxicity testing guidelines and research in immunotoxicity evaluation of food chemicals and new food proteins. Toxicol Pathol 28:467–478 7. Toxicological Principles for the Safety of Food Ingredients: Redbook 2000. Draft. Food and Drug Administration 8. Guidance for Industry: Immunotoxicology Evaluation of Investigational New Drugs. US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER). October 2002 9. Hastings KL (2002) Implications of the new FDA/ CDER Immunotoxicology guidance for drugs. Int Immunopharmacol 2:1613–1618 Relative Risk Regulatory T Cells A specific T cell subset controlling the response of other T cells by cell-cell contact and secretion of cytokines. Tolerance Suppressor Cells Hapten and Carrier 3 3 3 10. Committee for Proprietary Medicinal Products (CPMP). Note for Guidance on Repeated Dose Toxicity (CPMP/ SWP/1042/99). October 2000 11. Putman E, van Loveren H, Bode G et al. (2002) Assessment of the immunotoxic potential of human pharmaceuticals: a workshop report. Drug Info J 36: 417–427 12. Committee for Proprietary Medicinal Products (CPMP). Note for Guidance on the Quality, Preclinical and Clinical Aspects of Gene Transfer Medicinal Products (CPMP/BWP/3088/99). Draft version 13. International Trends in Immunotoxicity Studies of Medicinal Products. JPMA Drug Evaluation Committee Fundamental Research Group, Data 92. April 1999 14. Survey on Antigenicity and Immunotoxicity Studies of Medicinal Products. JPMA Drug Evaluation Committee Fundamental Research Group, Data 93. April 1999 15. Interim Draft Guidance for Immunotoxicity Testing. MHLW/JPMA, 2001 (unpublished) 16. ICH Topic S6: Preclinical Safety Evaluation of Biotechnology Derived Pharmaceuticals (CPMP/ICH/302/95). March 1998 17. Committee for Proprietary Medicinal Products (CPMP). Note for Guidance on Preclinical Pharmacological and Toxicological Testing of Vaccines (CPMP/ SWP/4654/95). June 1998 18. Guidance for Industry: Considerations for Reproductive Toxicity Studies for Preventive Vaccines for Infectious Disease Indications. US Department of Health and Human Services, Food and Drug Administration Center for Biologics Evaluation and Research. Draft version, August 2000 19. Guidance for Industry and FDA Reviewers: Immunotoxicology Testing Guidance. US Department of Health and Human Services, Food and Drug Administration Center for Devices and Radiological Health. 6 May 1999 20. Anderson JM, Langone JJ (1999) Issues and perspectives on the biocompatibility and immunotoxicity evaluation of implanted controlled release systems. J Control Rel 57:107–113 21. American Society for Testing and Materials: Standard Practice for Selecting Tests for Determining the Propensity of Materials to Cause Immunotoxicity. F1905–1998 22. American Society for Testing and Materials: Standard Practice for Evaluation of Immune Responses in Biocompatibility Testing Using ELISA Tests, Lymphocyte Proliferation, and Cell Migration. F1906–1998 23. OECD Guideline for the Testing of Chemicals 429: Skin Sensitisation: Local Lymph Node Assay. Adopted 24 April 2002 24. Health Effects Test Guidelines: OPPTS 870.2600 Skin Sensitization. US Environmental Protection Agency, March 2003 25. American Society for Testing and Materials: Standard Practice for Evaluation of Delayed Contact Hypersensitivity Using the Murine Local Lymph Node Assay (LLNA). F 2148–21401 555 Relative Risk Stephen B Pruett Department of Cellular Biology and Anatomy Louisiana State University Health Sciences Center Shreveport, Louisiana 71130 USA Synonyms None (in specific situations odds ratios can be numerically similar to relative risk but they are calculated differently). Definition Relative risk is the probability of an outcome in individuals exposed to a particular factor or condition divided by the probability of that outcome in individuals not exposed (1). Characteristics The characteristics of relative risk can best be understood by considering an example, as in Table 1. The relative risk of cancer for persons exposed to this toxicant is (25 ÷ 5025) ÷ (5 ÷ 5005) =0.004975 ÷ 0.000999=4.98. Thus, the risk of developing cancer is 4.98 times greater for the group exposed to the toxicant than for the nonexposed control group. Statistical analysis using a chi-square test or Fisher’s exact test is done to determine the statistical significance of this difference and to determine the confidence intervals. In this example, the P value is < 0.005 and the 95% confidence interval is 1.9–13.0. The fact that this interval excludes 1.0 (the value expected if there is no difference in risk between the exposed and unexposed groups) can also be used to demonstrate that the relative risk noted in this case is significant. Relative risk analysis is most often used with prospective studies (either cohort studies or randomized clinical trials). It is not suitable for case-control studies because these involve selection of cases on the basis of outcome, not exposure (1). The odds ratio can be R 556 RELISPOT Relative Risk. Table 1 An example of the characteristics of relative risk Cancer No cancer Total Toxicant exposure 25 5 000 5 025 No exposure 5 5 000 5 005 Total exposure 30 10 000 10 030 used for case-control studies. Interpretation of the biological relevance of relative risk data depends in part on an awareness of the distinction between relative risk and absolute risk. Increases in relative risk are more meaningful when the underlying absolute risks are relatively large than when they are small. Thus, a relative risk of 2.0 for an exposed population may cause relatively little concern if the frequency of the adverse outcome in the control population is 1:10 000 000, whereas more concern would be raised if the frequency of the adverse outcome is 1:100 in the control population. human populations) is still required for most toxicants in the risk assessment and regulatory process. References 1. Rosner B (2000) Fundamental Biostatistics. Duxbury Thompson Learning, Pacific Grove, CA, pp 54–58 2. Immune Deficiency Foundation. The Clinical Presentation of the Primary Immunodeficiency Diseases. A Primer for Physicians. The Laboratory Diagnosis of Immunodeficiency. http://www.primaryimmune.org Preclinical Relevance Relative risk analyses from human studies can be useful in risk assessment, particularly when toxicant exposure is associated with increased relative risk for a detrimental outcome. However, it should be noted that population sizes in published studies are generally not sufficient to permit small (but potentially meaningful) increases in risk to be demonstrated. For example, an increase from 2 cases of a particular outcome per 100 000 people to 4 cases per 100 000 people represents a relative risk of 2.0, but the 95% confidence interval is 0.37–10.9. Therefore, this two-fold change in risk is not statistically significant. An additional difficulty in using relative risk from human studies in the risk assessment process is that exposure assessment has generally received little attention, so that it is not usually possible to quantify the amount of exposure required to produce an adverse effect. Thus, extrapolation from animal studies (with uncertainty factors added for cross-species extrapolation and sensitive An experimental design in which each subject is measured at multiple time points. Measures over time within a subject will be correlated with one another and it is necessary to incorporate this into the analysis. Statistics in Immunotoxicology Replicate Cultures For many assays in cell biology (e.g. limiting dilution analysis), parallel cultures are set up under identical conditions to increase the precision of quantitative measurements. Limiting Dilution Analysis Reporter Antigen 3 Regulatory Environment Repeated Measures Design 3 Relative risk is commonly used in epidemiological studies to determine if exposure to toxicants is significantly associated with adverse health effects or changes in values for standard clinical tests (2). Enzyme-Linked Immunospot Assay 3 Relevance to Humans RELISPOT 3 Relative risk values could be calculated for most preclinical toxicology studies. However, this is usually not done, because other methods (e.g. analysis of variance with a post hoc test to compare means of multiple groups) are generally more appropriate and have greater statistical power. Reporter Antigen Popliteal Lymph Node Assay Reporter Antigen Popliteal Lymph Node Assay Reporter Antigen Popliteal Lymph Node Assay Raymond Pieters Head Immunotoxicology Institute for Risk Assessment Sciences (IRAS) Yalelaan 2 P.O. Box 80.176 3508 TD Utrecht The Netherlands Synonyms Popliteal lymph node assay, PLNA, reporter antigen, RA, RA-PLNA, ELISPOT Short Description Abbreviations RA-PLNA: RA-PLNA=reporter antigen popliteal lymph node assay TNP-OVA=trinitrophenyl-ovalbumin TNP-Ficoll=trinitrophenyl-Ficoll ASC=antibody secreting cells The reporter antigen-popliteal lymph node assay (RAPLNA) is a modification of the PLNA to determine compound-induced specific antibody responses (i.e. number of antibody secreting cells (ASC) by ELISPOT) to selected bystander antigens (1). This approach allows assessment of the nature and type of the immune response induced by chemicals in a straightforward and simple manner. The T-cell independent type 2 antigen TNP-Ficoll, which is susceptible to noncognate T cell help, is used as a reporter antigen (RA) that indicates or reports whether a chemical can induce neoantigen specific T cell help. By using the T cell-dependent antigen TNP-OVA (in a nonsensitizing concentration) as RA one can assess whether a chemical has adjuvant or sensitizing potential. By using the RA approach, characteristics of the chemically induced immune response (T cell dependency, adjuvant potential, type of immune response) can be determined without the need to know the neoantigens that are elicited by a compound and without the need to isolate or synthesize these neoantigens for assessment of anamnestic immune reactivity. So, as for the primary PLNA, the RA-PLNA allows fast screening of compounds for immunopotentiating effect, but in addition it enables discrimination between immunosensitizing and mere adjuvant or irritant potential of compounds. investigation; and the TNP-specific antibody secretion is determined in addition to cell numbers of the draining lymph nodes. Briefly, chemicals and RA (fixed final dose of 10 µg per mouse) are mixed in solution and injected into the hind footpad of a mouse. After 7 days the PLN is excised and used to prepare singlecell suspensions. PLN cells are counted and amounts of TNP-specific ASC in PLN suspensions are determined by ELISPOT. TNP-OVA and TNP-Ficoll are chosen because of their specific immunogenic properties. TNP-Ficoll is a T cell-independent type 2 antigen, that cannot be recognized by T cells, but that is very well capable of triggering B cells to produce immunoglobulin(Ig)M. Once triggered by TNP-Ficoll, B cells become susceptible to noncognate T cell help, meaning that such B cells will also produce switched isotypes such as IgG1 when soluble T cell help is available. Thus an IgG1 response to TNP-Ficoll indicates that T cells are activated (and possibly sensitized) and that these T cells recognize neoantigens induced by the chemical. These can be hapten-carrier complexes or other neoantigens (e.g. previously cryptic epitopes or hidden autoantigens). TNP-OVA is a protein antigen that can be recognized by T cells as well as B cells. The dose of TNP-OVA (10 µg per animal) that is used in the RA-PLNA is unable to elicit a specific immune response by itself. For a measurable immune response to TNP-OVA, extra or adjuvant signals are necessary. These adjuvant signals can be provided by any chemical that has some irritant or proinflammatory effect. In other words, a specific response against TNP-OVA indicates that the chemical is at least able to elicit an adjuvant signal. A specific antibody response against TNP-OVA does not, however, exclude sensitizing potential. But if an IgG1 response is elicited against TNP-OVA—but not against TNP-Ficoll—it is highly probable that the compound itself is not a sensitizer but that it has mere adjuvant potential. A chemical that does not eli- Characteristics The RA-PLNA differs from the PLNA in two ways: the RA are injected together with the chemical under 557 Reporter Antigen Popliteal Lymph Node Assay. Figure 1 Reporter antigens. R 558 Reporter Antigen Popliteal Lymph Node Assay cit an IgG response against any of the two RA can be considered as non-immunopotentiating. So, by combining the outcomes of the antibody (IgG1 or other switched isotypes) responses against TNP-Ficoll and TNP-OVA one can assess whether a chemical is a sensitizer (IgG1 to TNP-Ficoll and TNP-OVA) or a mere adjuvant (IgG1 to TNP-OVA but not to TNPFicoll), or is unable to elicit an immune response at all (no IgG1 to any of the RA) (see Figure 1). Interestingly, by changing the isotype specificity of the detection antibody in the ELISPOT assay (i.e. by using anti-IgG2a, anti-IgG2b and anti-IgE in addition to antiIgM and anti-IgG1) it is possible to determine the type (type 1 vs type 2) of the immune response. This can be done particularly well with TNP-OVA. This RA is also suitable to follow the immune response over a longer period of time (at least 4–5 weeks) and the type of memory response can be evaluated again without the need to know the relevant antigen induced by chemical exposure (2). Recent studies have been published in which the RAPLNA with TNP-Ficoll was combined with oral exposures to the drug diclofenac (3). Twenty days after single oral exposure to diclofenac, TNP-specific IgG1 responses were observed in the PLN upon footpad injection of a subsensitizing dose of diclofenac together with TNP-Ficoll. Hence, it appears possible to detect compound-specific anamnestic responses by using TNP-Ficoll which is susceptible to non-cognate neoantigen specific T cell help. It is important to note that coinjection of TNP-OVA or TNP-Ficoll did not appear to interfere with the type of immune response raised by the chemical. Pros and Cons The evident advantage of the RA-PLNA over the primary PLNA is that by the use of RA nonsensitizers can in principle be distinguished from nonsensitizing irritants. Moreover, the RA-PLNA provides a more robust indicator of immunostimulation (for chemicals the cell number varies from 1–2 × 106 to around 107 cells per PLN, whereas the number of IgG1 AFC varies from 0–10/106 to around 1300/106 PLN cells) and immunologically relevant information can be obtained in particular with respect to the type of immune response that is elicited. The kinetics of the immune response to TNP-OVA initiated by a certain chemical can be easily followed over a certain period of time. This allows easy verification of the adjuvant potential of a chemical by detecting memory responses to the RA. Apart from this, the RA-PLNA has pros and cons resembling those of the primary PLNA: it is a simple, straightforward, objective, and cheap test (despite the fact that it requires detection of ASC by ELISPOT) that allows fast screening of the immunostimulatory potential of compounds. Moreover, the outcome of the response depends likewise on the genetic background of the strain of mice used. Although false-positive chemicals (if they are so by irritancy) can be distinguished, false-negative pro-haptens remain undetectable without a metabolizing system. The RA-PLNA is also limited by the irrelevance of the route of exposure. Interestingly, however, the RA technique can be used in combination with exposure to compounds via the oral route. Predictivity The RA-PLNA was developed in 1996 and has been used primarily for fundamental research into immunomodulating capacity of low-molecular-weight chemicals. Hence, the number of compounds tested in the RA-PLNA is limited to around 20–30. Although not formally evaluated or validated, based on a comparison between two independent laboratories, the chemicals tested in the two laboratories showed similar outcomes (1,2,4). The RA-PLNA seems to be more robust then the primary PLNA (because of the use of an immunological parameter) so the selectivity of the assay may be improved by the use of RA. Relevance to Humans As for the primary PLNA, the RA-PLNA should be regarded as a screening test for the possibility of a compound to cause immunosensitization, and as a first step to evaluate whether the compound has also potential to stimulate the immune system via the relevant route of exposure. The relevance of the outcomes of the RA-PLNA might be higher because it gives substantially more information about the possible effect of the chemical exposure. Regulatory Environment The RA-PLNA was developed only 5–6 years ago and was mainly used to perform mechanistic studies. The RA-PLNA is regarded as a modification of the PLNA that has improved predictivity; it is therefore included in the ILSI-HESI initiative to be evaluated as a predictive test. References 1. Albers R, Broeders A, van der Pijl A, Seinen W, Pieters R (1997) The use of reporter antigens in the popliteal lymph node assay to assess immunomodulation by chemicals. Toxicol Appl Pharmacol 143:102–109 2. Albers R, de Heer C, Bol M, Bleumink R, Seinen W, Pieters R (1998) Selective immunomodulation by the autoimmunity-inducing xenobiotics streptozotocin and HgCl2. Eur J Immunol 28:1233–12342 3. Gutting BW, Updyke LW, Amacher DE (2002) BALB/c mice orally pretreated with diclofenac have augmented and accelerated PLNA responses to diclofenac. Toxicology 172:217–230 Respiratory Infections 4. Gutting BW, Schomaker SJ, Kaplan AH, Amacher DE (1999) A comparison of the direct and reporter antigen popliteal lymph node assay for the detection of immunomodulation by low molecular weight compounds. Toxicol Sci 51:71–79 Resident Macrophages Monocytes that migrate into normal tissues downregulate many activities and become resident macrophages which have reduced phagocytic and killing capacities but enhance signaling ability. Macrophage Activation 559 tagion, epidemics, consumption, air pollution, host defense systems Short Description Humanity has always been vulnerable to microbes that cause respiratory disease. Presently respiratory infection is the sixth leading cause of death in the USA—a situation which may intensify in coming years. Being aware of the health risk of respiratory infections has become more critical for five reasons: * the selection of resistant microbial flora due to the multitude of antimicrobial drugs currently available * the rapid international transport of microbes due to population migration * immunodeficiency diseases * increased mean life expectancy * the development of (formerly unavailable) surgical and systemic therapies for treating diseases. 3 3 Animal Models for Respiratory Hypersensitivity The severity and risk of infection varies with the virulence, antigenicity ( immunogenicity) and viability of the invading organism, the number of viable organisms at the target site, their ability to damage host tissue by the production of toxins, and the function of the individual’s normal microbial defenses. Microorganisms are highly versatile and widely distributed, occurring nearly everywhere in the environment. They are capable of replicating themselves or merely surviving in habitats that are extremely diverse and hostile. Ambient air that contains living organisms such as viruses, bacteria, fungi, protozoa, and algae (as well as products of their metabolism or their decomposition, such as toxins) is referred to as bioaerosols. When bioaerosols are inhaled and deposited in the respiratory tract, a normal host defense system exists to maintain health, and when this system is impaired, an individual’s risk of respiratory disease is increased. 3 3 3 Respiratory Hypersensitivity Test Animal Models for Respiratory Hypersensitivity 3 Respiratory Infections Donald E Gardner . Susan C Gardner Inhalation Toxicology Associates Inc. P.O. Box 97605 Raleigh, NC 27624 USA Synonyms Bioaerosols, pulmonary infections, pneumonia, influenza, bronchitis, common cold, SARS, airborne con- 3 The activation of oxidative metabolism of neutrophils, which is manifested by the production of highly reactive oxygen species, such as superoxide, hydroxyl radical and hydrogen peroxide. Respiratory burst is based on the activation of a multicomponent enzyme, NADPH-oxidase, in the neutrophil plasma membrane in response to various activators and during phagocytosis. Chemotaxis of Neutrophils 3 Respiratory Burst 3 Respiratory Allergy Assay Characteristics In the natural environment, healthy people exist in equilibrium with microorganisms. Microbes can be classified as pathogens, opportunists, or nonpathogens. Opportunistic microbes are organisms that normally are not capable of causing disease in a healthy immunocompetent person, but can cause disease in those with impaired host defense. The respiratory system is a most vulnerable target for such infectious agents because it is directly exposed to the external environment and has nearly four times the total surface area (70m2) as the combined total surface areas of the gastrointestinal tract and the skin. Although microbial uptake through ingestion and through the skin is generally intermittent, inhalation provides a continuous means of exposure. Thus, for airborne biological R Respiratory Infections 3 3 3 3 3 3 3 3 3 3 3 Environmental Factors Influencing Infectious Disease The presence of microbes in humans may be considered as the normal state, and the process of disease is a disturbance of the equilibrium between the host, the parasite, and the environment. The process of respira- tory infection and the subsequent disease involves the interaction of a host, a microbe and the environment. Thus, it becomes necessary that in addition to considering the virulence of the biological agent and the susceptibility of the host, attention must be given to a variety of environmental and physiological factors that might influence the course and severity of the disease. A person exposed to a combination of stresses, such as those of a physical or chemical nature, may be more susceptible to certain biological agents and thus may be at a greater risk of contacting a disease. A variety of gaseous and particulate airborne pollutants may adversely affect the normal functioning of the host’s respiratory defenses, which increases susceptibility to pulmonary infections. These include numerous inhaled metals (e.g. cadmium, lead, vanadium, nickel, manganese), gaseous pollutants (e.g. ozone, nitrogen dioxide, sulfur dioxide, phosgene, benzene, toluene, HCHO), particles (e.g. sulfuric acid), and complex mixtures (e.g. auto exhaust, tobacco smoke, wood smoke, fly ash) (1,2). Mechanisms by which these environmental factors could exacerbate the incidence and severity of infectious respiratory disease in individuals with normal immune function includes the following: * enhancing deposition * interfering with clearance and bactericidal activity * initiating inflammation leaving damaged epithelial tissue vulnerable to microbial invasion * by a mechanism that enhances delivery or infectivity of viruses, fungi and bacteria. Relevance to Humans The human relevance of understanding the source, transmission, pathogenesis, and the need for testing of bioaerosols is obvious. This has been clearly demonstrated with the recent epidemic of the new coronavirus that is most likely the cause of severe acute respiratory syndrome (SARS). Within 4 weeks of the first appearance of SARS, the microorganism infected people around the world. Success in controlling such epidiemics can be contributed to the worldwide global network of laboratories that pool resources to collaborate on such outbreaks. The 21st century faces a significant health threat from of the use of biological agents as terrorist weapons. The potential to cause large numbers of serious casualties among military forces and civilians provides an excellent reminder to medical planners of the limits of medicine. Biological weapons include any organism or toxin found in nature that can be used to incapacitate, kill, or impede people. Examples of human respiratory diseases associated with biological agents include anthrax, meningitis, plague, tularemia, brucellosis, smallpox, viral encephalitis and hemorrhagic 3 agents the respiratory system is a major route of entry into the body. An important distinction must be made between infection and disease. Infection implies that a microorganism has taken up residence in a host and is multiplying within that host—perhaps with no outward signs of disease. Thus it is possible to be infected with an agent, yet not have the disease (although disease may develop at a latter time). In contrast, those who appear “sick” are said to have a “disease”. Humans are the primary host for many microorganisms. After the microorganism has been inhaled and deposited in the respiratory tract, there are a number of elaborate defense mechanisms by which the respiratory system can protect this large surface area, including anatomical barriers (complicated shape of the nasal passages), physical clearance mechanisms (sneezing, coughing and ciliary activity), local antibody production (mainly immunoglobulin A, the preferred bacteriopsonin), production of interferons, proal antiproteases, antioxidants, fibronectin, teases, lactoferrin, phosholipids, phagocytic cells (alveolar macrophages and neutrophils) and immune effector cells (lymphocytes and natural killer cells). Together these defenses maintain the integrity of the respiratory system. Despite this protective system there are a number of ways that the inhaled invading organism can circumvent these defenses and cause disease either from deficiency in one or more of the mechanisms of defense or from exacerbation of virulence of the microorganisms such that the host defense system is overcome. Known factors which result in a weakened defense system include: * reduced physical removal of inhaled microorganism by the mucociliary mechanisms * dysfunction of the macrophages * alteration of the acellular lining material of the deep lung ( surfactant) or the mucus in the upper airways * presence of edema fluid or inflammatory exudates in the airways * pulmonary immunosuppression * influence of environmental pollutants * stress * preexisting disease * attenuated cytotoxic T cell function in malnourished children, alcoholics and elderly individuals * immunocompromised individuals such as HIV-positive patients or transplant patients receiving immunosuppressive therapy (1). 3 560 Respiratory Infections fever. A variety of microorganisms are capable of producing toxins such as aflatoxins, botulism, ricin, staphyloccal enterotoxin, mycotoxins and perfringen toxins. Toxin inhalation may cause acute illness with fever, sweating, muscle aches, rhinitis, and asthma. Currently it is estimated that about 17 countries are suspected of having offensive biological warfare programs, making the use of biological agents on military and civilian populations a greater threat than ever. Biological agents are easy to acquire, to synthesize, and to use. Only small quantities of microorganisms are needed to cause respiratory disease in people in metropolitan areas, so it is relatively easy to conceal, transport and disseminate them. These agents are difficult to detect or protect against since they are invisible, odorless, and tasteless, and their dispersal can be performed silently. 3 3 3 3 Assessment of Risk All microorganisms and chemical agents have the potential to be harmful under certain conditions of exposure. Examples of microorganisms causing respiratory infection or sensitization when inhaled are presented in Table 1. The important issue is not just of toxicity but also of risk. All humans accept some degree of risk in their daily activities. However, it is important to determine the probability that such an exposure will cause an adverse effect under actual conditions of human exposure. The National Academy of Science/National Research Council provides a structured approach to the process that has been widely used for assessing the risk of health effects resulting from exposure to chemical agents. There has been interest in developing similar risk assessment models to evaluate the likelihood of adverse human effects from exposure to infectious microorganisms. While the methods presently in use for chemicals are not directly appropriate for assessing risk from exposure to airborne pathogens, they do provide a conceptual framework for developing a similar process. Issues that are unique include assessment of the pathogen-host interaction, consideration of secondary spread, the possibility of shortterm and long-term protective immunity, and assessment of the conditions that might allow microorganisms to propagate. Thus, the development of a process for pathogenic risk assessment is complex and should be expected to consist of several interrelated components, which are conceptually distinct steps. Regulatory Environment US government agencies with regulatory responsibilities, including the Environmenta Protection Agency (EPA) and the Food and Drug Administration (FDA), have recommendations for guidelines for immunotoxicity testing strategies (3,4). These are discussed in 561 greater detail in other chapters. Such testing is intended to provide information on changes in the functioning of the immune system, which might occur as a result of exposure to a variety of agents. In the past, a battery of different assays, structured in a multitiered approach, has been used to assess immunotoxicity. Some of these assays included models for detecting respiratory susceptibility to bacterial and viral exposure. Respiratory susceptibility to infectious agents can best be measured if the immune system is asked to perform its normal functions (that is to defend against infectious agents) and to correlate changes in various host resistance animal models with changes in specific immune parameters. Since an increase in the incidence and/or severity of infection has been consistently identified as one of the hallmark indicators of immune malfunction, a great deal of research has been conducted to design and characterize such host resistance models (5). These studies have consistently indicated that changes in specific respiratory immune functional parameters are associated with the changes in host resistance models (making the host more susceptible to pulmonary infection). While these animal model systems are sensitive indicators for examining the enhancement of microbial infection of the lungs from exposure to airborne agents, it is now generally accepted that such host resistance models are not feasible choices as initial predictors of immunotoxicity because of their high complexity and cost. It is thought, therefore, that these models are best positioned in the second tier of a testing strategy. Control and Prevention The detection, prevention and management of airborne respiratory infectious disease depend on preventing the exposure to the biological agents. The ultimate aim must be to quickly identify the causative agent and to establish reliable approaches for prevention and control. A well-designed, well-implemented surveillance program can detect unusual clusters of respiratory disease, document an outbreak, estimate the magnitude of the problem, and identify factors responsible for its emergence. It is important to eliminate the reservoir of the microorganisms, to interrupt the transmission of the infection, and to increase the resistance of the individual to the microorganism. Personal hygiene and cleanliness in the living environment may lessen the spread of the disease, as well as using personal protective equipment in living and work places, proper disposal of waste (especially those suspected of microbial contamination), and the proper design and construction of buildings to avoid buildup of fungal growth. Special care is necessary in the case of immunocompromised and particularly sensitive or susceptible individuals. Drug-resistant bacterial, viral, and pro- R 562 Respiratory Infections Respiratory Infections. Table 1 Airborne microorganisms causing infection or sensitization Bacterial disease Causative Organism Primary Reservoir Pneumonia Nosocomial pneumonia Pneumonia Walking pneumonia Q fever Ornithosis, psittacosis Brucellosis* Legionnaires disease Tuberculosis Hypersensitivity pneumonitis Diphtheria Pertussis (whooping cough) Inhaled anthrax Bubonic plague* Tularemia Streptococcus pneumoniae Klebsiella pneumoniae Haemophilus influenzae Mycoplasma pneumoniae Coxiella burnetii Chlamydia psittaci Brucella melitensis Legionella pneumophila Mycobacterium tuberculosis Thermoactinomyces Corynebacterium diphtheriae Bordetella pertussis Bacillus anthracis Yersinia pestis Francisella tulaensis Humans Humans Humans Humans Animals Birds Animals Water Humans Water, soil, compost Humans Humans Animals Animals Animals Viral diseases Influenza Severe acute respiratory syndrome (SARS) Croup Bronchiolitis pneumonia Mumps* Measles Common cold Chicken pox* Small pox* Humans Influenza A, B and C viruses Humans, animals Coronaviruses Humans Parainfluenza viruses Humans Respiratory syncytial virus Humans Mumps virus Humans Rubella virus Rhinoviruses, coronaviruses, parainfluen- Humans za Humans Varicella zoster virus Humans Variola virus Fungal diseases Asthma, rhinitis Asthma, rhinitis Asthma, rhinitis Pulmonary aspergillosis Coccidioidomycosis Histoplasmosis Alternaria Cladosporium Penicillium Aspergillus Coccidioides immitis Histoplasma capsulatum Outdoor air, dead plants Outdoor air, dead plants Damp organic material Soil, compost Soil of arid regions Animals, soil, feathers Protozoa Algae Water reservoirs Water reservoirs Protozoan/ algal diseases Hypersensitivity pneumonitis Asthma, rhinitis * Disease transmitted via respiratory tract, but signs of infection and disease are seen elsewhere in body. tozoan pathogens pose a serious and growing problem for all people, regardless of their age, gender, or socioeconomic background. Microorganisms that are resistant to antibiotics cause the vast majority of infections that people acquire in hospitals. Drug resistance is accumulating and accelerating, thereby reducing the ability of drugs to combat infectious disease. While the emergence of microbial resistance cannot be stopped, the National Academy of Science/Institute of Medicine has addressed the urgency of this problem at the recent Forum on Emerging Infections. A number of specific suggestions were identified that will aid better understanding of microbial resistance, mitigating its impact on human health, thus transforming this growing threat into a manageable problem. References 1. Gardner DE (2001) Bioaerosols and disease. In: Bingham E, Cohrssen B, Powell CH (eds) Patty’s Industrial Hygiene and Toxicology, Volume 1, 5th ed. Wiley, New York, pp 679–711 2. Cohen MD, Zelikoff JT, Schlesinger RB (eds) (2000) Pulmonary Immunotoxicology, Kluwer, Norwell 3. US Food and Drug Administration (2002) Immunotoxicology Evaluation of Investigational New Drugs. FDA October 2002, pp 1–35 Rheumatoid Arthritis and Related Autoimmune Diseases, Animal Models 4. Environmental Protection Agency (1998) Health Effects Test Guidelines. OPPTS 870.7800 Immunotoxicity. EPA 712-C-96-351 October 1998, pp 1–11 5. Conn CA, Green FH, Nikula KJ (2000) Animal models of pulmonary infection in the compromised host. Inhal Toxicol 12:783–827 Responder Cell Specific cell types present in complex cell mixtures can be identified based on their functional response to specific stimulation. Limiting Dilution Analysis 563 Rheumatoid Arthritis and Related Autoimmune Diseases, Animal Models Jeanne M Soos Immunologic Toxicology, Preclinical Safety Assessment GlaxoSmithKline R&D 709 Swedeland Road P.O.Box 97605 King of Prussia, PA 19406-0939 USA Synonyms Arthritis models, autoimmune models 3 Short Description Responder Cell Frequency In limiting dilution analysis, the frequency of a specific cell type in a cell mixture is estimated based on its functional response to specific stimulation. Limiting Dilution Analysis 3 Reverse Enzyme-Linked Immunospot Assay RELISPOT is a synonym for ELISPOT. Enzyme-Linked Immunospot Assay 3 Reye’s Syndrome Fatal, fulminating hepatitis with cerebral edema. Anti-inflammatory (Nonsteroidal) Drugs 3 Rheumatic Fever Late complication of dermatological or pharyngeal infection. Some serologic subtypes of β-hemolytic streptococci lead to the production of antibodies against the bacterial cell wall protein (M protein). Some of these antibodies cross-react with myocardial sarcolemmal proteins, leading to carditis. Dermatological Infections Animal models of rheumatoid arthritis are experimental models of induced joint and digit inflammation that can be utilized to investigate the mechanisms contributing to arthritic inflammation, to investigate potential therapies for rheumatoid arthritis, and to assess the potential for substances to either induce or downregulate autoimmune inflammatory responses. Autoimmune arthritis can be induced by injection or immunization by several different types of antigens in multiple susceptible strains of mice, rats, and higher animals (1). Characteristics Each of the arthritis models described below is characterized by induction of disease through immunization with either a self antigen or injection with a mixed antigen preparation. Assessment of arthritis in animal models is achieved through visual inspection of the front and hind paws. Inflammation can occur in the ankle and throughout the digits depending on the severity of disease. The scoring system for assessing the severity of inflammation is presented in Table 1. The scores for each joint are added and thus the maximum score for an individual animal is 16. Rheumatoid Arthritis and Related Autoimmune Diseases, Animal Models. Table 1 Scoring system for visual evaluation of experimental arthritis Score Pathology on Visual Inspection 0 Normal size and structure of paw 1 Swelling observed in a single digit 2 Swelling observed in more than one digit 3 Swelling observed in the joint 4 Complete distention and swelling of digits and joint R 3 564 Rheumatoid Arthritis and Related Autoimmune Diseases, Animal Models Additional methods for assessing development of disease in models of arthritis include the measurement of paw thickness using a caliper, mercury or water plethysmography, histopathology of the joint, and radiographic evaluation of the joint. Collagen-Induced Arthritis (CIA) Collagen-induced arthritis can be induced in a variety of rodent strains as well as nonhuman primates. Type II collagen, the specific self antigen used for disease induction, is a major component of cartilage. Similar to human rheumatoid arthritis, susceptibility to collagen-induced arthritis is linked to the expression of certain major histocompatability complex (MHC) class II molecules (2). Disease is monophasic and can result in full resolution of disease or ankylosis of the joint. Pathogenesis of disease in this model involves both T cell and B cell responses. Adjuvant Arthritis (AA) Adjuvant arthritis can be induced in a variety of rat strains with the Lewis rat most commonly used. Rats are immunized with heat-killed Mycobacterium tuberculosis, strain H37Ra, in incomplete Freund's adjuvant and evaluated over time for development of disease. The disease tends to be monophasic and often results in irreversible joint ankylosis. Disease can also be induced by adoptive transfer of mycobacteria-specific T cells or lymph node cells of immunized rats. Streptococcal Cell Wall Arthritis (SCW) Streptococcal cell wall arthritis is inducible in a wide variety of rat strains and, depending on the strain, the susceptibility to disease development and severity of disease can vary. Streptococcal cell wall group A peptidoglycan-polysaccharide polymer preparation isolated from Streptococcus pyogenes cell walls is injected intraperitoneally for development of disease. The course of the disease is characterized by acute onset during the 48 h after injection followed by a chronic phase. Measurement of Cellular Responses and Cytokines in Arthritis Models Humoral responses can be evaluated by measuring antibody titers to the antigens used for induction of disease in the models described above. T cell responses can be measured by cellular proliferation assays as well as by the generation of antigen-specific T cell lines and clones. Cytokine analysis can be a valuable method for evaluating the mechanisms for modulation of disease. A list of the characteristics of each of these models is presented in Table 2. Pros and Cons There are some advantages of the individual models of arthritis. The use of a specific self antigen, such as type II collagen in the CIA model, allows for the dissection of multiple cellular inflammatory mechanisms by the methods described above. Induction of disease in the SCW arthritis model illustrates molecular mimicry and allows for study of the initial pathogenesis and potential triggers of the autoimmune response (3). Also the SCW is a chronic model of disease that more closely reflects the course of rheumatoid arthritis in humans. Disadvantages also exist for these models. The course of disease is limited only to an acute phase, with no observations of a longer, chronic phase in the collagen-induced and adjuvant arthritis models. Further, for both the collagen-induced and SCW models, great attention must be paid to the quality and purity of the antigen preparation used to induce disease. Predictivity These models are the methods of choice for initial pharmacological studies, having good predictive value. However they may not be fully predictive of what may be observed in humans. For example, blockade of an inflammatory cytokine was shown to be therapeutic in animal models of autoimmunity (4,5) while blockade of that same cytokine induced autoimmunity in clinical studies (6,7). Immunological mechanisms leading to autoimmunity in humans are more complicated and less well understood than the prevention of autoimmunity (i.e. by blockade of T helper type 1 cytokines) in these animal models. For immunotoxicology, these models of inflammation provide a valuable means for evaluating potential for proinflammatory activities of new drugs and substances. Relevance to Humans Animal models of arthritis serve as a means for evaluating new therapies for human rheumatoid arthritis. Regulatory Environment The field of autoimmunity and the use of autoimmune models such as rheumatoid arthritis do not require regulation, but the value of these models is recognized for understanding the mechanisms of immunotoxicologic potential and for risk assessment by the FDA in the guidance for Immunotoxicology Evaluation of Investigational New Drugs. Relevant Guidance FDA (CDER) Immunotoxicology Evaluation of Investigational New Drugs, 2002 Ricin 565 Rheumatoid Arthritis and Related Autoimmune Diseases, Animal Models. Table 2 Characteristics of the most widely employed animal models of experimental rheumatoid arthritis Animal Models Species/Strains Collagen-induced arthritis Chicken or bovine type II Mouse: primarily DBA/1, strains of the I-Aq and I-Ar collagen class II allelles Rat: variety of strains from multiple class II Rt alleles Monophasic Mouse: days 21–35 Rat: high responder days 8–10 Rat: low responder days 30–60 Adjuvant arthritis Lewis rat Monophasic Days 10–17 Heat-killed Mycobacterium tuberculosis H37Ra Disease Course Streptococcal cell wall Biphasic group A peptidoglycan-polysaccharide polymers 3 3 Rhinitis Inflammation of the nasal mucus membrane, marked by sneezing, lacrimation and watery mucus. Respiratory Infections 3 Ribonucleic Acid (RNA) A biomolecule that has an informational, structural, and enzymic role. The structure is of ribose units joined in the 3' and 5' positions through a phosphodiester linkage with a purine or pyrimidine base attached to the 1' position. (RNA = ribonucleic acid). Southern and Northern Blotting 3 Ricin A highly toxic lectin and hemagglutin occurring in seeds of castor beans. Used as a chemical warfare agent. Respiratory Infections 3 A common inflammatory disease caused by an autoimmune reaction directed to joints. It is mostly mediated by humoral immune reactions and immune complex deposits. So-called rheumatoid factors, i.e. autoantibodies against the constant region (Fc) of antibodies, have been described for this disease for the first time. This chronic potentially disabling arthritic condition with a female predominance, is character- 3 Rheumatoid Arthritis (RA) ized by peripheral symmetric polyarthritis with or without other associated systemic involvements, occurring predominantly in individuals who are HLADR4/DR1 positive. Hypersensitivity Reactions Systemic Autoimmunity Fatty Acids and the Immune System Complement Deficiencies Molecular Mimicry Cyclosporin A 3 1. Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Stober W (eds) (1991) Current Protocols in Immunology. John Wiley and Sons, New York 2. Wooley PH, Luthra HS, Stuart JM, Cavid CS (1981) Type II collagen-induced arthritis in mice. I: Major histocompatibility complex linkage and antibody correlates. J Exp Med 154:688–700 3. Taylor JE, Ross DA, Goodacre JA (1994) Group A streptococcal antigens and superantigens in the pathogenesis of autoimmune arthritis. Eur J Clin Invest 24:511–521 4. Mori L, Iselin S, de Libero G, Lesslauer W (1996) Attenuation of collagen-induced arthritis in 55-kDa TNF receptor type 1 (TNFR1)-IgG1-treated and TNFR1deficient mice. J Immunol 157:3178–3182 5. Korner H, Goodsall AL, Lemckert FA et al. (1995) Unimpaired autoreactive T-cell traffic within the central nervous system during tumor necrosis factor receptormediated inhibition of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 92:11066–11070 6. Sicotte NL, Voskuhl RR (2001) Onset of multiple sclerosis associated with anti-TNF therapy. Neurology 57:1885–1888 7. Mohan N, Edwards ET, Cupps TR et al. (2001) Demyelination occurring during anti-tumor necrosis factor alpha therapy for inflammatory arthritides. Arthritis Rheumatol 44:2862–2869 Acute phase: 48 hours Chronic phase: days 10– 21 with continued disease for months 3 References Induction of Disease 3 Streptococcal Lewis rat primarily cell wall arMultiple rat strains with thritis varying susceptibility Antigen for Immunization R 566 Risk Assessment Risk Assessment Statistics in Immunotoxicology 3 Rodent Immune System, Development of the Kenneth S. Landreth Department of Microbiology, Immunology, and Cell Biology West Virginia University Health Sciences Center Morgantown, WV 26506 USA Synonyms Murine immune system, mouse immune system, rat immune system Definition Our understanding of cells and tissues that make up the immune system of vertebrates came from seminal studies of avian species. However, rodents have become the animal model of choice for studies of immunocompetence, and most of our knowledge of the development of the immune system has come from studies using mice or rats. The rodent immune system develops through a set of critical windows of vulnerability during embryonic and adult life that can be used for evaluating effects of environmental exposure to potentially toxic compounds (1). Most of the information in this review comes from studies of mice and, in all cases tested, closely parallels that found in other rodents, including rats. Characteristics Embryonic development. Embryonic development of the immune system in rodents initiates with formation of multipotential hematopoietic stem cells (HSC) in intraembryonic spanchnoplure surrounding the heart and in association with endothelial cells of the extraembryonic yolk sac (2). Hematopoietic stem cells undergo a temporal migration from intra-embryonic mesenchyme to fetal liver, fetal spleen, and ultimately to final residence in bone marrow and thymus: organs which continue to produce immunocompetent cells throughout life (Figure 1). HSC first appear in embryonic mice during the 7th day of gestation. These cells develop in both intraembryonic splanchnopleuric mesenschyme surrounding the heart, a tissue often identified as the aorto-gonadomesonephric region (AGM), and in extraembryonic blood islands of the yolk sac (3). However, HSC in these two embryonic tissues differ dramatically in their developmental fate. Intraembryonic stem cells, but not those that arise in the yolk sac, contribute to sustained blood cell development and functional immune-responsive cells in postnatal rodents. The standard experimental assay for HSC has been the in vivo spleen colony forming cell (CFU-S) assay. This assay relies on the unique ability of rodent stem cells to migrate to the spleen after adoptive transfer and to initiate formation of macroscopic colonies of hematopoietic cells that are clonally derived. More recent in vitro assays for multipotential hematopoietic cells have been developed and enumerate cells capable of forming colonies in a semisolid matrix that contain multiple blood cell lineages. The initial period of stem cell formation for the hematopoietic system culminates as newly developed stem cells migrate to the embryonic liver and spleen. At approximately day 10 of gestation in mice, HSC relocate from the AGM to the developing fetal liver. In fetal liver, HSC develop into more differentiated and lineage-restricted stem cells. These lineage-restricted stem cells further differentiate in this tissue site to form more mature progenitor cells which retain the ability to proliferate but are more restricted in their developmental potential. Lineage-restricted stem cells are operationally defined by their proliferation and/or differentiation in response to specific hematopoietic cytokines. Progenitor cells are routinely enumerated using in vitrocolony forming unit (CFU) cell assays. The availability of recombinant cytokines and use of these assays have been invaluable in enumerating specific progenitor cells in embryonic tissues that are otherwise indistinguishable by morphologic analysis. These assays are central to any study of direct effects of toxic compounds on hematopoietic tissues and blood cell formation in the developing embryo or in postnatal animals. Fetal Liver. Fetal liver continues to be the principal hemato-lymphopoietic organ until near the end of gestation, however, few morphologically or functionally identifiable mature leukocytes are found in the embryo until near the time of birth (4). B lymphocyte production in the rodent fetal liver has been well characterized and serves as a prototype of fetal development of the immune system (5). Cells with immunoglobulin (Ig) gene rearrangements are first found in the liver on gestational day 11 and increase rapidly to easily detectable numbers by gestational day 13. Cells with cell surface Ig (B lymphocytes) are not detected in fetal liver until day 18 of gestation, and remain at low frequency until birth. Numbers of hematopoietic cells decline in the fetal liver as the bone marrow assumes primary hematopoietic function at gestational day 18. HSC and lineage-restricted lymphoid progenitor cells are found in the developing spleen on gesta- 3 Rodent Immune System, Development of the 567 Rodent Immune System, Development of the. Figure 1 Organs which continue to produce immunocompetent cells throughout life. Adapted from: Dietert RR et al. (2000) Environ Health Perspect 108 [Suppl 3]:483–490 tional day 13, approximately the same time they are found in the fetal liver and remain detectable in that tissue until a few weeks after birth. Unlike the bone marrow, lymphopoiesis rapidly wanes in the spleen after birth and can not be demonstrated in adult mice. Thymus. Organogenesis of the thymus initiates from the 3rd and 4th pharyngeal pouches in mice on gestational day 11. The thymus is immediately colonized by immigrant HSC, which are detectable on day 11 of gestation. The thymus continues to be a source of lymphoid cells (T lymphocytes) in postnatal rodents until somewhat after sexual maturity when the thymus regress and ceases function. Lymph nodes. Lymph nodes form in the developing embryo by endothelial budding of the venous circulatory system, a process that initiates on gestational day 10.5 in mice. The formation of these organs is dependent on interaction of immature lymphoid cells with developing endothelial cells. Peyer’s patches develop from clusters of cells on the proximal end of the intestine on gestational day 15.5 and nasopharyngeal lymphoid tissues form after birth in mice. Bone marrow. Long bones of the embryo mineralize, and the central marrow cavity is excavated to create a marrow cavity on gestational day 17.5 in mice. This new site is immediately populated by hematopoietic cells derived from AGM (not yolk sac) (2). This population of cells establishes the hematopoietic bone marrow which serves as a reserve of HSC, blood cell development, and production of immune-responsive cells for the remainder of postnatal life. Relevance to humans Observations made on development of human lymphoid tissues and cells suggest that the temporal sequence of events described here for rodents is largely duplicated in human development (Figure 1). However, there are notable differences between the gestational appearance of immune function in rodents and humans. In general, immune responsive cells appear relatively earlier in gestation in human embryos, and tissues of the immune system are more mature at birth than in rodents. Of particular importance, cytokines that stimulate lymphoid cell proliferation and differentiation are conserved between humans and rodent species and, in many cases, retain sufficient homology to be active on cells from either species. Regulatory Environment Rodents are the preferred model for studies of immunology and immunotoxicology and most regulatory testing is carried out on these species. Despite the fact that we know significantly more about the development of the immune system in mice, rats continue to be a preferred model for immunotoxicology testing for historical rather than scientific reasons. References 1. Dietert RR, Etzel RA, Chen D, Halonen M, Holladay S, Jarabek AM, Landreth K, Pedan D, Pinkerton K, Smialowicz RJ, Zoetis T (2000) Workshop to identify critical windows of exposure for children’s health: immune and respiratory systems work group summary. Environ Health Perspect 108 [Suppl 3]:483–490 2. Cumano A, Godin I (2001) Pluripotent hematopoietic stem cell development during embryogenesis. Curr Opin Immunol 13:166–171 3. Metcalf D, Moore MAS (1971) Haemopoietic Cells. In: Neuberger A, Tatum EL (eds) Frontiers of Biology, Vol. 24. North-Holland, Amsterdam, pp 172–271 4. Landreth KS (2002) Critical windows in development of the rodent immune system. Hum Exp Toxicol 21:493– 498 5. Landreth KS (1993) B lymphocyte development as a developmental process. In: Cooper EL, Nisbet-Brown E (eds) Developmental Immunology. Oxford University Press, New York, pp 238–273 R 568 Rodents, Inbred Strains Rodents, Inbred Strains Ina Hagelschuer PH-R ZfV, Geb. 516 Bayer HealthCare AG Aprather Weg 18 D-42096 Wuppertal Germany Synonyms Inbred strains, inbreds, genetically defined rodents Definition General items Most of the current definitions of inbred strains are established from rats and mice. These rodents have short-generation intervals and sufficient numbers of offspring to allow the application of close inbreeding. In contrast, only a few strains being inbred by definition (see below) are available from hamsters or guinea pigs. Inbred strain A strain shall be regarded as inbred when it has been mated brother × sister (hereafter called b×s) for 20 or more consecutive generations. Parent × offspring matings may substitute b×s matings provided that, in the case of consecutive parent × offspring matings, the mating in each case is to the younger of the two parents. Inbred strains are designated by three to five capital letters (for example the mouse strain CBA or the rat strain BN). Before creating new designations relevant databases have to be checked in order to avoid duplications. Present information on nomenclature rules can be drawn from the Jax Mice Catalogue (1). * a combination of both if more than one substrain is developed in the same laboratory, e.g. CF/1Ztm, CF/2Ztm.CF/4Ztm for Central Laboratory Animal Facility, Medical University Hannover. Coisogenic strain Inbred strains are coisogenic to each other if they differ in only one allelic character. This difference can be only caused by a mutation and subsequent fixation of the mutated allele. Designation of a coisogenic strain Symbol of the background strain followed by the differentiating allele in italic letters, separated by a hyphen, e.g. C.B-Igh-1b/IcrTac-Prkdcscid (formerly C.B17-Prkdcscid). This strain is an example of a mutation within a congenic strain. scid * symbol: Prkdc * symbol name: severe combined immune deficiency (the mutation scid) * gene name: protein kinase, DNA-activated catalytic polypeptide (Prkdc) * symbol description: mutation in the gene encoding the catalytic subunit of DNA-activated protein kinase, Prkd. Arose in the C.B-17 congenic strain. Substrain Inbred strains are divided into substrains when there are known or assumed genetic differences due to residual heterozygosity during colony set-up, mutation, or genetic contamination. These reasons are likely when: * the strains are separated before inbreeding generation F40 * the present strain has been bred separately for 50 or more generations * genetic differences have been proven. Congenic strain A congenic strain is developed by transfer of a chromosomal segment (consisting of the differentiating locus and flanking genes) from a donor strain to another strain (inbred background or recipient strain). The genetic background of the initial crosses have to be purified by at least 10 backcrosses with the recipient strain. Afterwards the original inbred strain and the congenic strain (recipient strain) should only differ in this introduced segment. A congenic strain should be mated b×s after having finished the backcross process (see Fig. 1). The designation of a congenic strain contains the symbols or abbreviations of the recipient and donor strain, separated by a dot. The transferred gene segment is added in italic letters separated by a hyphen. Thus, for C.B-Igh-1b/IcrTac (formerly.C.B-17) * donor strain: C57BL/Ka strain (B) * background strain: BALB/c, (C) b * symbol name: immunglobulin H-1 b * gene name: heavy chain allele (Igh-1 ) b * [C.B-17 = BALB/c.C57BL/Ka-Igh-1 ] (number 17 originated from the backcross number 17). Designation of a substrain Parental strain or symbol for differentiation (examples of symbols): * numbers, e.g. DBA/1 or DBA/2 * laboratory codes, e.g. A/J for Jackson Laboratory For B10.129P-H12b/(6M)SnJ * donor strain: 129P3/J * background strain: C57BL/10n * symbol name: histocompatibility 12b * gene name: histocompatibility 12. Rodents, Inbred Strains F1-hybrid The cross of two inbred strains leads to an F1 hybrid. All F1 animals of the same parental strains are genetically identical. They are heterozygous at all loci having different alleles in the parental strains. F1 hybrids share the advantages of inbred strains. In addition they are robust against environmental influences resulting in low quantitative character variability. An important limitation is the discontinuation of breeding. The designation of a F1-hybrid consists at first of the symbol of the female parent followed by that of the male parent. Short or full symbols may be used. For example, B6C3F1 is the result of a female C57BL/6 mouse crossed to a C3H male mouse. Characteristics Advantages of inbred strains Inbred animals of the same strain can be regarded as identical twins and can be reproduced unlimited. Long-term genetic stability Inbred strains can be assumed to be genetically constant for a long period of time, supposed they are correctly bred and genetically monitored. Under these conditions background data on strain characteristics may be comparable for many years. 569 Isogenicity All individuals of a strain are isogenic. Thus skin grafts and tumors may be transplanted within an inbred strain without immunological rejection. Genetic data can be accumulated within the same strains. Of special interest for immunotoxicologists are data on major histocompatibility complex (MHC) (Fig. 2). Homozygosity Inbreds are homozygous at virtually all loci, and thus will breed true within the strain. International distribution Most of the used inbred strains have an international distribution, thus the genetic results of research can be easily compared. Identification/monitoring Inbreds can be identified by their strain-specific genetic profiles consisting of DNA polymorphisms, immunologic markers (e.g. MHC haplotypes in the mouse; RT1 haplotypes in the rat) and biochemical markers. The regular monitoring of genetic profiles can reduce the risk of unnoticed genetical contamination. The strain monitoring has to be performed of course by competent laboratories. Uniformity Genetic variation is reduced to nearly zero within strains by constant inbreeding. The use of inbred strains enable a much better standardization of the test conditions. Application of inbreds can improve the quality, the repeatability and comparability of results. The extent of analysis may be reduced or kept at a minimum by selecting an appropriate genetic model. Such a principle is in line with animal welfare legislation calling for reduction and refinement of animal experiments. Individuality Each inbred strain represents an unique genotype. This genotype can lead to a phenotype of biomedical interest (for example, inbreds with high or low tumor incidence, or high or low disease resistance). Limitations of inbred strains Individuality Each inbred strain represents only one genotype. To extrapolate experimental results different inbred strains have to be considered, ideally including also their F1 hybrids. Rodents, Inbred Strains. Figure 1 Congonic strains. From: Cruse JM, Lewis RE (2004) Atlas of Immunology. CRC Press, Boca Raton. Isogenicity and uniformity The variability of quantitative characters within a single strain is exclusively due to non-genetic factors, like environmental or methodological factors. R 570 Rodents, Inbred Strains Rodents, Inbred Strains. Figure 2 H-2 histocompatibility system is the major histocompatibility complex in the mouse. From: Cruse JM, Lewis RE (2004) Atlas of Immunology. CRC Press, Boca Raton. Examples of commonly used inbred strains in the field of immunotoxicology Brown Norway rat (BN) (RT-1 haplotype n) The BN rat is characterized by high basal level of serum IgE which can be induced massively by immunization with proteins. The inducibility of the IgE response is currently under evaluation as an indicator for allergic asthma and for chemically induced autoimmunity. As a reason for this high responsiveness the reduced antioxidant levels in the BAL cells is considered, resulting in reduced ability to adapt to oxidative stress induced by allergen induction. In 2002 Vohr et al reported on a model that uses the reactions of regional lymph node as indicators for the induction of respiratory allergy. The BN rat was also found to be a suitable model in a modified local lymph node assay (LLNA), self-limiting increase of IgE, reduced endogenous antioxidant level in BALF, and food allergy test. 3 B6C3F1 and C57BL/6 (h-2 haplotype b/k and b) Historically both strains have been used extensively in the National Toxicology Program to set up an immunotoxicologic database which consists of a vast variety of tests of over 50 compounds (for a review article see Luster et al 1994 ). Both strains gave acceptable results in comparison study of mouse strains in a LLNA. BALB/c (h-2 haplotype d) This strain was used in the first LLNA. Its feasibility has been confirmed recently by Woolhiser et al in 2000, and Hüsler et al in 2003. BALB/c mice are IgE high responders and they are occasionally used for investigations of the regulation and induction of this antibody subclass. Because of this they have also been the object of intensive investigations for estab- lishing short-term models for the differentiation between respiratory and (skin) contact allergy. CBA/Ca and CBA/J (h-2 haplotype k) The strain CBA/Ca was recommended by Kimber and Weisenberger in 1989 for the murine LLNA as a result of comparison of four murine strains. Young adult male or female mice of CBA/Ca (or CBA/J strain in the USA) are also recommended for the LLNA in the OECD guideline 429, 2002. The Fischer rat (F344-RT1 haplotype lv1), Lewis rat (LEW-RT-1 haplotype l), and A/J-mouse (h-2 haplotype a) are also widely used in different immunotoxicity studies. Preclinical Relevance Inbred strains have made an essential contribution to biomedical research. Much progress in research, especially in the field of immunology, has followed from the development of inbred strains. Use of inbred strains enables much better standardization of the conditions. Inbreds help to improve the quality and reproduction of the results obtainable. A comparability of the results is given. Selecting an appropriate genetic model reduces the analysis needed, and may even keep it to a minimum. These points are in line with the animal welfare legislation calling for reduction and refinement of animal experiments. On the other hand, the increased variability of immune responses of non-inbred strains (so-called outbred stocks) does reflect the human situation in a more realistic way. Results obtained by the use of outbred animals may thus have a better impact on risk assessment. Therefore, there is still debate about which of the strains mentioned are to be used for immunotoxicologic investigations. The various inbred rat and mouse strains show differences in their immunological reactions. Many differences occur especially in the RTqPCR field of sensitivity (from high-responders to non-responders). Other 'normal' immunoreactions can also differ, like the plaque-forming cell assay (PFCA) against sheep erythrocytes (SRBC). Undesired hyperreactions or autoimmune reactions may also depend on the genetic background, as well as host-resistance analysis. So it is essential that a rat or mouse strain must be checked for its immunological reactivity before using it for immunotoxicologic examinations. In addition, positive controls have to be established. 571 3. Festing M (1993) International index of laboratory animals, 6th ed. The British Library, London 4. Foster HL, Small JD, Fox JG (1981) The mouse in biomedical research, Volume I: History, genetics, and wild mice. Academic Press, New York 5. Hedrich HH (1990) Genetic monitoring of inbred strains of rats. Gustav Fischer Verlag Stuttgart, New York 6. Heinecke H (1989) Angewandte Versuchtierkunde. VEB Gustav Fischer Verlag, Jena 7. Klein J (1986) Natural history of the major histocompatibility comple. John Wiley and Sons, New York 8. Krinke GJ (2000) The laboratory rat. Academic Press, New York Relevance to Humans With the exception of the above-mentioned OECD guideline 429 there are no recommendations for the use of special inbred or outbred strains in other immunotoxicity guidelines. These techniques are used to identify or isolate particles or cells bound by indicator cells or erythrocytes. For example, mixing sheep erythrocytes with human blood cells results in rosetting of human T cells surrounding the sheep erythrocytes. This erythrocyte (E) rosette was the first technique in separating T from B cells. The receptor responsible for this binding is the CD2 molecule on T cells. The rosettes are named after the central particle, i.e. E-rosette (see above), erythrocyte antibody (EA) rosette, or erythrocyte antibody complement (EAC) rosette. Rosetting Techniques RT1.B, RT1.D (Rat) 3 Regulatory Environment Rosetting Techniques 3 Inbred strains serve as models of human diseases in various disciplines like immunobiology, transplantation medicine, autoimmunity, and oncology. Inbred mice and rats of one strain can be seen as identical twins. The rat can also be used as an experimental model for nutrition research, with a reliable correlation of approximately 0.98 between rat and human in the digestibility of nutrients. Research applications of the rat seems to be dominated by assigning function to the complete genomic sequence, particularly with respect to those regions involved in common human diseases. All in all, the rat offers the best 'functionally' characterized mammalian model system. Antigen Presentation via MHC Class II Molecules RTqPCR References 3 1. Jax Mice Catalogue (2001) http://www.informatics.jax. org/menus/strain_menu.shtml 2. Baker HJ, Lindsey JR, Weisbroth SH (1979) The laboratory rat, Vol. 1: Biology and diseases. Academic Press, New York Polymerase Chain Reaction (PCR) R


![Early immune system exposure linked to chronic disease[1]](http://s3.studylib.net/store/data/007014068_1-a55ba8d48625efeb17f49a28d1617f27-300x300.png)