THE OCCURRENCE OF ANGUILLICOLA CRASSUS (KUWAHAR, NIMI, AND HAGAKI,
advertisement

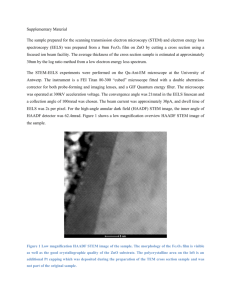
THE OCCURRENCE OF ANGUILLICOLA CRASSUS (KUWAHAR, NIMI, AND HAGAKI, 1974), AN INTRODUCED NEMATODE, IN AN UNEXPLOITED WESTERN IRISH EEL POPULATION M. Morrissey and T.K. McCarthy ABSTRACT The presence of Anguillicola crassus , an introduced Asian parasite of eels, into south Connemara is reported. European eels Anguilla anguilla were found to be infected in the three basins of varying salinity of Lough Ahalia, but eels sampled in the nearby marine Camus Bay were uninfected. Variations in infection parameters were analysed. It was concluded that the parasite is a recent introduction to the river system. The highest prevalence (63.1%) and mean intensity (4.22) of infection in the lower, most saline, basin of the lough suggest that the parasite was introduced to the River Screebe system at that location. Potential dispersal mechanisms by which the parasite may have been introduced to such a relatively isolated location and to such apparently unexploited eel populations are discussed. M. Morrissey (corresponding author e-mail: morrissey. michelle@gmail.com) and T.K. McCarthy, Department of Zoology, National University of Ireland, Galway, Ireland. Received 21 April 2006. Accepted 10 September 2006. Published 12 February 2007. BIOLOGY AND INTRODUCTION The swimbladder-inhabiting nematode Anguillicola crassus (Kuwahar, Nimi, and Hagaki, 1974) is an eelspecific parasite, accidentally introduced into Europe in the early 1980s through uncontrolled transfer of live eels from Eastern Asia (Neumann 1985). Presence of the parasite can induce pathogenic changes in the swimbladder and reduce the ability of the eel to withstand additional stressors (Kirk 2003). Anguillicola crassus was first recorded in Ireland from eels captured in the Waterford Estuary in 1997 (McCarthy et al . 1999). Since then it has become well established in several commercially exploited Irish eel fisheries such as those on the Shannon and Erne river systems (Evans and Matthews 1999; McCarthy et al . 1999), Lough Corrib, Lough Neagh and the Nore, Barrow, Suir and Slaney Rivers (McCarthy et al., in press). As part of a larger investigation of the population biology of European eels (Anguilla anguilla L.) in Irish marine and mixohaline waters, samples from unexploited eel populations in south Connemara were found to be infected with A. crassus . In this paper we detail and analyse the A. crassus infection parameters recorded. STUDY AREA European eels Anguilla anguilla were sampled along a short /2km salinity gradient in Lough Ahalia and ENVIRONMENT: PROCEEDINGS OF THE in Camus Bay, western Ireland (Fig. 1). Lough Ahalia, a coastal lagoonal lake (Healy 2003), consists of three basins forming the lower reaches of the River Screebe catchment. The system flows into the Atlantic via Camus bay. The upper basin of Lough Ahalia (surface area /40ha, mean depth 1.7m) is freshwater (0.1); the middle basin (surface area /60ha, mean depth 2.5m) is brackish (12), receiving bi-monthly tidal inputs of saline water (i.e. on spring tides); the lower basin (surface area /25ha, mean depth 1.6m) is more influenced by the tides and receives a twice-daily influx of tidal water, which leads to near full marine salinities (25). Camus Bay is fully saline (34). The site was chosen because of the relatively steep salinity gradient over a small distance, but more importantly, because there is no exploitation of the eel population. MATERIALS AND METHODS Samples of yellow eel were obtained from the four salinity zones (upper basin, middle basin, lower basin and Camus Bay) during October 2004, using unbaited fyke nets. Fyke nets were set at dusk and lifted the following morning. Eels were transferred on ice to the lab where they were measured (total length, to the nearest millimetre), and weighed (to the nearest gram). An index of condition was calculated using Fulton’s condition factor ROYAL IRISH ACADEMY, VOL. 107B, NO. 1, 13 18 (2007). # ROYAL IRISH ACADEMY 13 BIOLOGY Fig. 1 * AND ENVIRONMENT Map of Ireland showing the location of Lough Ahalia and Camus Bay. K (100W LT3). Sex determination was by macroscopic examination of the gonads (Beullens et al . 1997). The swimbladder was removed, and A. crassus present in the swimbladder lumen were removed and counted as described by Gollock et al . (2004) and Molnár et al. (1993). Parasitological terms (prevalence and mean intensity) are used in accordance with Bush et al. (1997). Prevalences were compared by Fishers exact test, and mean intensities were compared using a Bootstrap 2 sample test (Rózsa et al . 2000). In both cases differences were considered significant if P B/0.05. Table 1 * RESULTS Descriptive statistics for length and weight and condition of eels from the four sites are shown in Table 1. Analysis of variance showed that mean log-transformed (log (x)) lengths of eels in the four sites were significantly different (F /4.002, P B/ 0.05) and post hoc analysis (LSD) showed that the differences occurred in eels between Camus Bay and the lower and upper basins of Lough Ahalia (P B/0.05). There was a significant difference in log-transformed weight between the four sites Summary statistics for eel length, weight and condition from the upper, middle and lower basins of Lough Ahalia and Camus Bay. Site Upper Middle Lower Camus Bay Total No. of eels Length (mm) Min. Max. Mean SD 30 312 694 431.03 109.04 121 300 855 447.76 109.24 65 257 690 418.35 75.51 85 290 755 471.32 100.46 301 257 855 446.4 101.67 Weight (g) Min. Max. Mean SD 55.2 685 182.34 171.21 53.4 1392 220.37 246.05 43.8 673 145.40 93.11 38.8 896 214.25 163.44 38.8 1392 198.66 193.08 Condition Min. Max. Mean SD 0.136 0.221 0.1845 0.0196 0.129 0.365 0.1957 0.0386 0.139 0.56 0.1821 0.0384 0.132 0.221 0.1753 0.0187 0.129 0.456 0.1859 0.0334 14 THE OCCURRENCE OF (ANOVA; F /3.296, P B/0.05), and the LSD test showed the lower basin of Lough Ahalia was significantly different to the middle basin and Camus Bay (P/ 0.05). There was a significant difference in square-root-transformed condition between the four sites (ANOVA; F/4.021, P B/ 0.05), and the LSD test showed that the differences occurred in eels from Camus Bay and those from the lower and upper basins of Lough Ahalia (P B/0.05). Details of the infection levels of A. crassus recorded in eels in the four sampling sites expressed as prevalence (% infected), mean intensity (mean number per infected host) and maximum burden are given in Table 2. Anguillicola crassus was not found in eels examined from Camus Bay. Prevalence and mean intensities were highest in the lower basin of Lough Ahalia and decreased from lower to middle to upper basins of Lough Ahalia. There was a significant difference in both prevalence (P B/0.05) and mean intensity (P B/ 0.05) between the middle and lower basin. Sex was determined for all eels (n /301). An overall predominance of females (88%) was observed (n /265). There was no significant difference in sex ratios between the 4 sites (x2 / 7.63, d.f. /3, P /0.054). Table 2 also displays mean intensity of infection, prevalence and maximum burden of A. crassus in male and female eels for the total sample and from the middle basin and the lower basin, where levels of infection were highest. Overall, levels of infection were higher in males than females. Levels of infection were compared between males and females in the middle and lower basins. In the middle basin there was a significant difference in prevalence (P B/0.05), with males having a higher prevalence than females but not a greater mean intensity (P/ Table 2 * ANGUILLICOLA CRASSUS 0.7475). In the lower basin there was no significant difference between the sexes with regard to prevalence (P/0.304) or mean intensity (P/ 0.3215). Variation in infection intensity between length classes is shown in Fig. 2. Spearman rank order correlations showed a highly significant negative correlation between length of eel and number of A. crassus present in the middle basin (rs / /0.252, P B/0.05) and no correlation in the lower basin (rs / 0.070, P/0.578). Though not systematically investigated during the present study, damage to the swimbladder wall was frequently observed in eels infected with A. crassus . There was no significant correlation between Fulton’s condition factor (K) of eels and their parasite burdens in either the middle basin (rs / /0.018, P /0.845) or lower basin (rs / 0.201, P /0.108). Because smaller eels in the middle basin tended to have more parasites, both male and females in the smallest length class ( B/400mm) from this basin were compared to investigate whether there was a relationship between sex and intensity of infection. A significant difference was found between males (n /15) and females (n /32) B/400mm, with males having a higher intensity of infection than females in the same size range (x2 / 10.885 df /4, P B/0.05). The variance-to-mean ratio (/s2 =x) of parasite abundance was calculated to provide an index of the degree of dispersion of A. crassus among individual host eels. A ratio of /1 indicates overdispersion, a ratio equal to 1 indicates random distribution in the parasite, and a ratio of B/1 indicates underdispersion (Anderson and Gordon 1982). The variance-tomean ratios were both /1, indicating overdispersion (middle basin s2 =x /2.28 and lower basin s2 =x / 5.63). The abundance of A. crassus in eels displayed a negative binomial distribution in both the middle Anguillicola crassus maximum burden, % prevalence and mean intensity for total sample of eels (in bold) and male and females separately, sampled from the upper, middle and lower basins of Lough Ahalia and Camus Bay. Site Upper Female Male Middle Female Male Lower Female Male Camus Bay Female Male No. of eels Infected eels Max. burden Prevalence (%) Mean intensity 30 24 6 121 106 15 65 54 11 85 81 4 2 2 0 36 25 11 41 36 5 0 0 0 1 1 0 7 7 5 17 16 17 0 0 0 6.7 6.7 0 29.8 23.58 77.33 63.1 66.66 45.45 0 0 0 1.5 1.5 0 1.75 1.8 1.64 4.22 3.83 7.0 0 0 0 15 BIOLOGY AND ENVIRONMENT Mean number of parasite ± 95%CI 4.5 Middle Basin 4 Lower Basin 3.5 3 2.5 2 1.5 1 0.5 0 200-299 Fig. 2 * 300-399 400-499 500-599 600-699 Length class (mm) DISCUSSION Anguillicola crassus , accidentally introduced from Asia in the 1980s, has been progressively expanding its range in the eel-inhabited inland waters of Europe. Its dissemination has been linked to the extensive commercial transport of live eels and re-stocking of eel fisheries with infected fish, as well as natural movements of infected eels (Kennedy and Fitch 1990; Wickströem et al. 1998; Kirk 2003). Its arrival in Ireland in the 1990s and its subsequent colonisation of major eel fisheries has been documented by McCarthy et al . (in press). The discovery that eels in the relatively isolated lower River Screebe area are now infected suggests that the species is progressively extending its range in Ireland and that a better understanding of its dispersal routes and mechanisms is needed. The parasite is recognised as being pathogenic in the European eel, with adverse effects on affected swimbladders and on various other aspects of eel physiology having been recorded (Kirk 2003). Despite the damage caused by A. crassus to swimbladders of eels noted in the (a) 100 Observed Expected 75 50 25 (b) 100 Observed Expected n = 65 75 50 25 0 0 0 16 present study, infected eels generally appeared to be in good condition. Thus, in the present study, no correlation was observed between Fulton’s condition factor (K) and parasite burden in Lough Ahalia. However, such eels are likely to have their capacity to migrate to the Sargasso spawning area adversely affected by their A. crassus infections. As demonstrated in experimental studies on Dutch silver eels, A. crassus can reduce the swimming speeds and increase the metabolic cost of silver eel migration (EELREP 2005). The observations made on the infection levels of A. crassus in Lough Ahalia eels (Table 2) suggest that they were initially introduced to the mixohaline lower basin and that, as indicated by the progressively lower infection parameters in the middle and upper basins, the parasite has been gradually spreading towards the freshwater areas upstream. A car parking area adjacent to Screebe Bridge, where Lough Ahalia discharge connects to Camus Bay, may have enabled someone transporting live eels to exchange water in their transport tanks. This practice was commonly undertaken by commercial eel dealers in the past. Alternatively, even though no authorisations have been granted by the fishery owner or licenses issued for that area by the Western Regional Fishery Board for over eight % frequency % frequency n = 121 * 800-899 Variation of Anguillicola crassus intensity with eel length class in middle and lower basin. (k/0.465) and lower basin (k/0.556) (Fig. 3a and 3b). Fig. 3 700-799 2 4 6 8 10 12 Number of A. crassus 14 16 0 2 4 6 8 10 12 Number of A. crassus 14 16 Percentage frequency of Anguillicola crassus in eels from (a) the middle and (b) the lower basin of Lough Ahalia. THE OCCURRENCE OF years, it is known that some illegal fishing for eels occasionally occurs in the area. It is possible that transport of eel catches between river basins by such fishermen resulted in the spread of A. crassus to the Screebe system. Dispersal by natural mechanisms from River Corrib basin, where A. crassus is well established, seems less likely. A cormorant colony located on an island in the upper basin of Lough Ahalia might have a role in extending the distribution of A. crassus through fish regurgitation, involving infected eels or paratenic hosts, as has been shown in Polish studies (Wlasow et al. 1998). However, observations on flight direction adopted by foraging birds suggest that they do not regularly forage in the Corrib system. Likewise, though theoretically possible, it is unlikely that the initial colonisation of the Screebe River system resulted from natural movements of infected eels or paratenic hosts. The need to consider such possibilities is suggested by recent observations on the variety of migratory strategies adopted by eel species in which local movements between waters of differing salinities can occur throughout the yellow eel foraging phase of the eel life cycle (Harrod et al. 2005). Eels from Camus Bay examined in this study (Table 2) were found not to be infected with A. crassus , suggesting that colonisation of the Lough Ahalia eel populations did not occur through eels migrating into the system from the marine environment. The spread of A. crassus within the system may have been facilitated by the natural movement of eels between the Lough Ahalia basins (Harrod et al. 2005). Parasite infections typically vary among size classes of their fish hosts, and differences in transmission rates often reflect ontogenic trophic niche shifts. Various studies have shown that larger eels tend to have higher numbers of A. crassus than smaller eels (Audenaert et al . 2003; Schabuss et al. 2005). However, in the Lough Ahalia middle basin samples, A. crassus abundance was negatively correlated with eel size. This may reflect unusual features of the habitat and the trophic ecology of its eels. Piscivory is typical of eels greater than 50cm in length in many freshwater habitats. However, limited observations on stomach contents of the eels captured in the marine and mixohaline habitats sampled in Camus Bay and Lough Ahalia suggests that larger prey items are more likely to be crabs, such as the euryhaline Carcinus maenas . Larger eels typically do not feed directly on copepods, such as those bearing infective A. crassus larvae, but can be infected by preying on fish fry containing numerous recently ingested copepods (Kirk 2003). Unlike most European habitats colonised by A. crassus , the Screebe system is characterised by having relatively species-poor fish assemblages. In this respect it is typical of smaller western Irish ANGUILLICOLA CRASSUS rivers and lakes, where cyprinids and other nonindigenous coarse fish are generally absent. Results of stable isotope analyses of eels from the three basins of Lough Ahalia suggested that the trophic niche occupied by eels in the middle basin was narrower than either of the other basins (Harrod et al. 2005). This may be due to limited fish prey, although it may also reflect food web changes associated with the presence of salmon-rearing cages in that lake basin. Interestingly males had more parasites than females of the same size class in the middle basin. However, whether this resulted from differences in trophic ecology, inter-habitat movements or other factors is unclear. Several of the euryhaline species present in the lower basin and in nearby Camus Bay, are listed among the more than 37 species recorded as paratenic hosts for A. crassus (Höglund and Thomas 1992; Szekely 1994; Kirk 2003). The higher prevalence of A. crassus in the lower basin of Lough Ahalia may be due to ingestion of infected paratenic hosts by eels. The absence of A. crassus in the eels sampled in Camus Bay may be explained by research that has shown that the parasite prefers lower salinities (Kennedy and Fitch 1990). However, adult nematodes can survive within eels in the marine environment by osmoconforming with the blood plasma of the eel (Kirk et al . 2002), so their absence from Camus Bay may not be entirely due to salinity. A further explanation may be the lack of available intermediate hosts, which is a known factor to limiting the spread of A. crassus (Kirk et al. 2000). Concerns have been expressed about the negative impact A. crassus may have on the capacity of eels to undertake their oceanic spawning migrations. It has been suggested that A. crassus parasitism may be contributing to the dramatic decline that has occurred in European eel stocks in recent decades (Køie 1991). Therefore, more systematic analyses of dispersal and pathogenic effects are needed. As indicated by Kirk 2003, relatively little is known about A. crassus in coastal and brackish water habitats, with the exception of studies on exploited Baltic eel populations and some other local studies on estuarine habitats. McCarthy et al. (in press) reviewed available information on parasites of Irish eels and highlighted the need for parasitological research on eels in marine and mixohaline waters. The results of the present study, while providing new information on this species in Ireland and contributing to knowledge of eel ecology in mixohaline waters, also suggest that further investigations on A. crassus transmission in such habitats are needed. 17 BIOLOGY AND ACKNOWLEDGEMENTS This study was funded as part of the HEA-PTRLI Cycle 3 project Population Biology of Eels in Marine and Mixohaline Waters. We would like to thank Dr Chris Harrod for help with fieldwork and the Screebe Fishery for allowing us to sample the eel populations of Lough Ahalia. REFERENCES Anderson, R.M. and Gordon, D.M. 1982 Processes influencing the distribution of parasite numbers within host populations with special emphasis on parasite-induced host mortalities. Parasitology 85, 373 98. Audenaert, V., Huyse, T., Goemans, G., Belpaire, C. and Volckaert, F.A.M. 2003 Spatio-temporal dynamics of the parasitic nematode Anguillicola crassus in Flanders, Belgium. Diseases of Aquatic Organisms 56, 223 33. Beullens, K., Eding, E.H., Gilson, P., Ollevier, F., Komen, J. and Richter, C.J.J. 1997 Gonadal differentiation, intersexuality and sex ratios of European eel (Anguilla anguilla ) maintained in captivity. Aquaculture 153, 135 50. Bush, A.O., Lafferty, K.D., Lotz, J.M. and Shostak, A.W. 1997 Parasitology meets ecology on its own terms: Margolis et al . revisited. Journal of Parasitology 83, 575 83. EELREP 2005 Final report: estimation of the reproduction capacity of European eel. pp. 272. (EU-project EELREP (Q5RS-2001-01836)). Evans, D.W. and Matthews, M.A. 1999 Anguillicola crassus (nematoda, Dracunculoidea); first documented record of this swimbladder parasite in eels in Ireland. Journal of Fish Biology 55, 665 8. Gollock, M.J., Kennedy, C.R., Quabius, S.E. and Brown, A.J. 2004 The effect of parasitism of European eels with the nematode, Anguillicola crassus , on the impact of netting and arial exposure. Aquaculture 233, 45 54. Harrod, C., Grey, J., McCarthy, T.K. and Morrissey, M. 2005 Stable isotope analyses provide new insights into ecological plasticity in a mixohaline population of European eel. Oecologia 144, 673 83. Healy, B. 2003 Coastal lagoons. In M.L. Otte (ed.), Wetlands of Ireland. Distribution, ecology and economic value , 51 78. Dublin. University College Dublin. Höglund, J. and Thomas, K. 1992 The black goby Gobius niger as a potential paratenic host for the parasitic nematode Anguillicola crassus in a thermal effluent of the Baltic. Diseases of aquatic organisms 13, 175 80. Kennedy, C.R. and Fitch, D.J. 1990 Colonization, larval survival and epidemology of the nematode 18 ENVIRONMENT Anguillacola crassus, parasitic in the eel, Anguilla anguilla , in Britain. Journal of Fish Biology 36, 117 31. Kirk, R.S. 2003 The impact of Anguillicola crassus on European eels. Fisheries Management and Ecology 10, 385 94. Kirk, R.S., Lewis, J. W. and Kennedy, C.R. 2000 Survival and transmission of Anguillicola crassus Kuwahara, Niimi and Itagaki, 1974 (Nematoda) in seawater eels. Parasitology 120, 289 95. Kirk, R.S., Morritt, D., Lewis, J.W. and Kennedy, C.R. 2002 The osmotic relationship of the swimbladder nematode Anguillicola crassus with seawater eels. Parasitology 124, 339 47. Køie, M. 1991 Swimbladder nematodes (Anguillicola spp.) and gill monogeneans (Pseudodactylogyrus spp.) parasitic on the European eel (Anguilla anguilla ). Journal du Conseil International pour l’Exploration de la Mer 47, 391 8. McCarthy, T.K., Creed, K., Naughton, O., Cullen, P. and Copley, L. in press The metazoan parasites of eels (Anguilla anguilla ) in Ireland: zoogeographical, ecological and fishery management perspectives. In J. Casselman and D. Cairns (eds), American Fisheries Society Symposium series. McCarthy, T.K., Cullen, P. and O’Connor, W. 1999 The biology and management of River Shannon eel populations. Fisheries Bulletin (Dublin) 17. Molnár, K., Baska, F., Csaba, G., Glávatis, R. and Székely, C. 1993 Pathological and histopathological studies of the swimbladder of eels Anguilla anguilla infected by Anguillicola crassus (Nematoda:Dranunculoidea). Diseases of Aquatic Organisms 15, 41 50. Neumann, W. 1985 Schwimmblasenparasit Anguillicola bei Aalen. Fischer Teichwirt 11, 322. Rózsa, L., Reiczigel, J. and Majoros, G. 2000 Quantifying parasites in samples of hosts. Journal of Parasitology 86, 228 32. Schabuss, M., Kennedy, C.R., Konecny, R., Grillitsch, B., Reckendorfer, W., Schiemer, F. and Herzig, A. 2005 Dynamics and predicted decline of Anguillicola crassus infection in European eels, Anguilla anguilla , in Neusiedler See, Austria. Journal of Helminthology 79, 159 67. Szekely, C.S. 1994 Paratenic hosts for the parasitic nematode Anguillicola crassus in Lake Balaton, Hungary. Diseases of Aquatic Organisms 18, 11 20. Wickströem, H., Clevestam, P. and Höglund, J. 1998 The spreading of Anguillicola crassus in freshwater lakes in Sweden. Bulletin Francais de la Pêche et de la Pisciculture 349, 215 21. Wlasow, T., Gomulka, P., Martyniak, A., Boron, S., Hliwa, P., Terelecki, J. and Szymanska, U. 1998 Anguillicola crassus larvae in cormorant’s prey fish in Vistula Lagoon, Poland. Bulletin Francais de la Pêche et de la Pisciculture 349, 223 7.