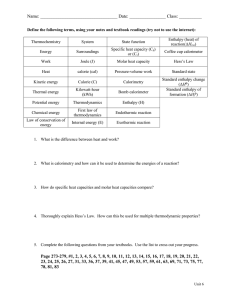
5.2
advertisement
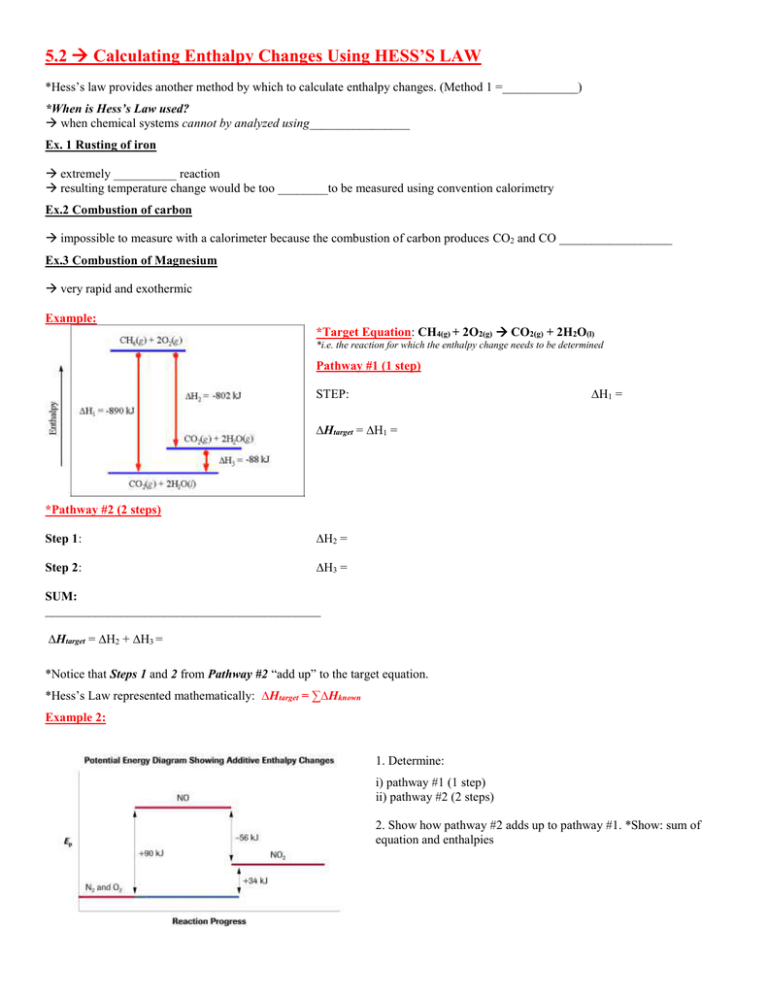
5.2 Calculating Enthalpy Changes Using HESS’S LAW *Hess’s law provides another method by which to calculate enthalpy changes. (Method 1 =____________) *When is Hess’s Law used? when chemical systems cannot by analyzed using________________ Ex. 1 Rusting of iron extremely __________ reaction resulting temperature change would be too ________to be measured using convention calorimetry Ex.2 Combustion of carbon impossible to measure with a calorimeter because the combustion of carbon produces CO2 and CO __________________ Ex.3 Combustion of Magnesium very rapid and exothermic Example: *Target Equation: CH4(g) + 2O2(g) CO2(g) + 2H2O(l) *i.e. the reaction for which the enthalpy change needs to be determined Pathway #1 (1 step) ∆H1 = STEP: ∆Htarget = ∆H1 = *Pathway #2 (2 steps) Step 1: ∆H2 = Step 2: ∆H3 = SUM: ____________________________________________ ∆Htarget = ∆H2 + ∆H3 = *Notice that Steps 1 and 2 from Pathway #2 “add up” to the target equation. *Hess’s Law represented mathematically: ∆Htarget = ∑∆Hknown Example 2: 1. Determine: i) pathway #1 (1 step) ii) pathway #2 (2 steps) 2. Show how pathway #2 adds up to pathway #1. *Show: sum of equation and enthalpies PRACTICE Questions: *Use Hess’s Law to determine the enthalpy change associated with the indicated reaction 1. Show how the molar enthalpy of formation for gaseous ammonia, NH3(g), can be calculated from the following reactions. *First, indicate the Target Equation: 3H2(g) + N2(g) 2NH3(g) or [3/2H2(g) + 1/2N2(g) NH3(g) ∆Hf = ?] Known equation #1: NH3(g) + Known equation #2: H2(g) + 3 3 1 O2(g) H2O(g) + N2(g) 4 2 2 1 O2(g) H2O(g) 2 ∆H1 = -316.4 kJ ∆H2 = -241.6 kJ 2. Nitromethane is a rapid-burning fuel often used in dragsters where rate, not energy yield is important. 4CH3NO2(g) + 3O2(g) 4CO2(g) + 2N2(g) + 6H2O(g) ΔH˚ = ? (a) Use Hess’s law to determine the enthalpy change for the reaction above using the following thermochemical equations. (b) Determine the molar enthalpy of combustion of nitromethane. C(s) + O2(g) CO2(g) 2H2(g) + O2(g) 2H2O(g) 2C(s) + 3H2(g) + 2O2(g) + N2(g) 2CH3NO2(g) ΔH˚ = -393 kJ ΔH˚ = -484 kJ ΔH˚ = -226 kJ b) Determine the amount of energy released when 50.0 kg of nitromethane is combusted. RICHARD THORNLEY: HESS’S LAW: https://www.youtube.com/watch?v=u9mRp0WJUzI