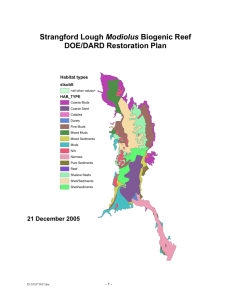
Modiolus Restoration Research Project: Prepared by:
advertisement

Modiolus Restoration Research Project: Final Report and Recommendations (20th May 2011) Prepared by: D Roberts, L Allcock, J M Fariñas-Franco, E Gorman, C A Maggs, A M Mahon, D Smyth, E Strain, and C D Wilson 1 Contents Executive Summary ............................................................................................................ 8 Summary of key findings ............................................................................................................... 10 Changes in the distribution, density and condition of M. modiolus reefs in Strangford Lough (Undertakings 1, 2 and 7) .............................................................................................................. 10 Small-scale temporal and spatial variability in ‘good’ and ‘poor’ M. modiolus reefs (Undertakings 4 and 5) ......................................................................................................................................... 10 Potential for natural recovery of M. modiolus reefs (Undertaking 6) .......................................... 11 Identifying suitable sites for restoration: habitat suitability modelling for Modiolus modiolus, in Strangford Lough (Undertakings 9 and 10) ................................................................................... 12 Intervention (Undertakings 11, 12 & 13) ...................................................................................... 13 Projection for recovery of ‘Favourable Conservation Status’ (Undertaking 8)............................. 15 Recommendations (Undertaking 3) ............................................................................................. 20 PROTECTION ................................................................................................................................. 20 INTERVENTION .............................................................................................................................. 21 MONITORING ................................................................................................................................ 22 1.0 Introduction ........................................................................................................... 25 1.1 The Modiolus Restoration Research Project ................................................................ 25 1.2 Background ........................................................................................................................ 28 1.3 Rationale ............................................................................................................................ 31 TECHNICAL REPORTS ..................................................................................................... 34 2.0 Changes in the distribution, density and condition of Modiolus modiolus communities in Strangford Lough (Undertakings 1, 2 and 7) ........................................ 35 2.1 Summary ............................................................................................................................ 35 2.2 Introduction ........................................................................................................................ 36 2.3 Methods .............................................................................................................................. 37 2.3.1 Remotely Operated Vehicle surveys (2008-2010) .......................................................... 37 2.3.2 Dive surveys (2008-2010) ................................................................................................ 38 2 2.3.3 Ultra Short Baseline acoustic surveys (2008-2010)......................................................... 39 2.3.4 Historical records (1954-2007)........................................................................................ 40 2.3.5 Analyses........................................................................................................................... 42 2.4 Results ................................................................................................................................ 45 2.4.1 Distribution of M. modiolus ............................................................................................ 45 2.4.2 Condition of M. modiolus communities .......................................................................... 49 2.3.3 Percentage cover of dead M. modiolus .......................................................................... 52 2.3.4 Historical trends in M. modiolus distribution and densities in Strangford Lough .......... 54 2.4 Discussion .......................................................................................................................... 55 2.5 Conclusions ....................................................................................................................... 57 3.0 Small-scale temporal and spatial variability in ‘good’ and ‘poor’ M. modiolus reefs (Undertakings 4 and 5) ............................................................................................ 58 3.1 Summary ............................................................................................................................ 58 3.2 Introduction ........................................................................................................................ 59 3.3 Methods .............................................................................................................................. 60 3.3.1 Site characteristics .......................................................................................................... 60 3.3.2 Sampling strategy ............................................................................................................ 62 3.3.3 Monitoring of M. modiolus and epifauna: in situ counts and photo quadrats ............... 62 3.3.4 Monitoring of M. modiolus epifauna, crevice and sediment infauna: core sampling .... 62 3.3.5 Analyses........................................................................................................................... 63 3.4 Results ................................................................................................................................ 64 3.4.1 Density of M. modiolus ................................................................................................... 64 3.4.2 Photo quadrat monitoring of epifauna ........................................................................... 65 3.4.3 Core sampling.................................................................................................................. 71 3.5 4.0 4.1 Discussion .......................................................................................................................... 76 Potential for natural recovery of M. modiolus communities (Undertaking 6).... 78 Summary ............................................................................................................................ 78 4.2 Introduction ............................................................................................................................... 79 3 4.3 Methods .............................................................................................................................. 80 4.3.1 Transect surveys – Changes in frequency of occurrence of M. modiolus between 2003 and 2010 ....................................................................................................................................... 80 4.3.2 Transect surveys – Changes in M. modiolus abundance and communities between 2003 and 2007 ....................................................................................................................................... 81 4.3.3 Removal quadrats – Changes in M. modiolus densities and communities between 2003 and 2010 ....................................................................................................................................... 82 4.3.4 4.4 Analyses........................................................................................................................... 83 Results ................................................................................................................................ 85 4.4.1 Transect surveys – Changes in frequency of occurrence of M. modiolus between 2003 and 2010 ....................................................................................................................................... 85 4.3.3 Removal quadrats – Changes in density of M. modiolus between 2003 and 2010 ........ 92 4.4.3 Epifauna, and crevice and sediment infauna in quadrats ............................................... 94 4.5 In situ observations ......................................................................................................... 102 4.5.1 North Basin (SS.SBR.SMus.ModCvar) ............................................................................ 102 4.5.2 South basin (SS.SBR.SMus.Mod.HAs) ............................................................................ 102 4.5.3 Historical sites within the range of M. modiolus in Strangford Lough .......................... 103 4.6 Discussion ........................................................................................................................ 104 4.7 Conclusions ..................................................................................................................... 105 5.0 Identifying suitable sites for restoration: habitat suitability modelling for M. modiolus, in Strangford Lough (Undertakings 9 and 10) ............................................. 106 5.1 Summary .......................................................................................................................... 106 5.2 Introduction ...................................................................................................................... 108 5.3 Methods ............................................................................................................................ 110 5.3.1 Landscape parameterization ......................................................................................... 110 5.3.2 Statistical analyses ........................................................................................................ 113 5.4 Results .............................................................................................................................. 114 5.4.1 SS.SBR.SMus.ModCvar .................................................................................................. 114 5.4.2 SS.SBR.SMus.ModHAs/ModT ........................................................................................ 114 4 5.4.3 5.5 6.0 Both basins .................................................................................................................... 115 Discussion ........................................................................................................................ 119 Intervention action .............................................................................................. 121 6.1 Translocation or restructuring of scattered, un-clumped adult Modiolus modiolus and subsequent monitoring (Undertaking 11).............................................. 122 6.1.1 Summary .......................................................................................................................... 122 6.1.2 Introduction ...................................................................................................................... 124 6.1.3 Aims and objectives........................................................................................................ 125 6.1.4 Materials and Methods ................................................................................................... 125 6.1.4.1 Site selection ............................................................................................................. 125 6.1.4.2 Survey methodology ................................................................................................. 127 6.1.4.3 Site selection survey results...................................................................................... 128 6.1.4.4 Artificial reef experimental design............................................................................ 129 6.1.4.5 Deployment of cultch ............................................................................................... 130 6.1.4.6 Translocation of adult Modiolus modiolus ............................................................... 133 6.1.4.7 Monitoring ................................................................................................................ 134 6.1.4.8 Additional clumping behaviour experiment ............................................................. 136 6.1.5 Results .............................................................................................................................. 136 6.1.5.1 Epifaunal community succession .............................................................................. 136 6.1.5.2 Effect of relief on M. modiolus survival .................................................................... 141 6.1.6 Discussion ........................................................................................................................ 142 6.1.7 Conclusions ..................................................................................................................... 144 6.2 Provision of suitable substrata for spat settlement and subsequent monitoring (Undertaking 12).............................................................................................................. 145 6.2.1 Summary .......................................................................................................................... 145 6.2.2 Introduction ...................................................................................................................... 146 6.2.3 Materials and methods ................................................................................................... 146 6.2.3.1 Natural recruitment .................................................................................................. 147 5 6.2.3.2 Shell aging methods .................................................................................................. 147 6.2.3.3 Spat settlement......................................................................................................... 149 6.2.4 Results .............................................................................................................................. 153 6.2.4.1 Population structure and natural recruitment ......................................................... 153 6.2.4.2 Growth and age-frequency distributions.................................................................. 156 6.2.4.3 Spat settlement......................................................................................................... 157 6.2.4.4 Spat settlement......................................................................................................... 159 6.2.5 Discussion ........................................................................................................................ 160 6.2.5.1 Population structure and natural recruitment ......................................................... 160 6.2.5.2 Substrate preference ................................................................................................ 161 6.3 Pilot Modiolus modiolus hatchery cultivation (Undertaking 13) ...................... 163 6.3.1 Summary .......................................................................................................................... 163 6.3.2 Introduction ...................................................................................................................... 164 6.3.3 Materials and Methods ................................................................................................... 165 6.3.3.1 Production of micro-algae. ....................................................................................... 166 6.3.3.2 Conditioning of brood-stock and induction of broodstock spawning ...................... 168 6.3.3.3 Larval husbandry ....................................................................................................... 169 6.3.3.4 Intermediate cultivation ........................................................................................... 172 6.3.4 Results .............................................................................................................................. 172 6.3.4.1 Broodstock size frequency ........................................................................................ 172 6.3.4.2 Broodstock response to supplemental algal diet ..................................................... 173 6.3.4.3 Description of spawning and the larval cycle ........................................................... 174 6.3.4.4 Effect of diet in larval growth and survival ............................................................... 176 6.3.4.5 Settlement ................................................................................................................ 178 6.3.5 Discussion ........................................................................................................................ 179 6.3.5.1 Hatchery cultivation of M. modiolus ........................................................................ 179 6.3.5.2 Economics of seed mussel production ..................................................................... 181 6 6.3.6 7. Conclusions ................................................................................................................... 182 Projection for recovery of ‘Favourable Conservation status’ (Undertaking 8) .... 183 7.1 Summary .......................................................................................................................... 183 7.2 Introduction ...................................................................................................................... 184 7.3 Current conservation status ................................................................................................. 184 8 Recommendations (Undertaking 3) ........................................................................ 189 8.1 Options .................................................................................................................................... 189 8.2 Recommendations................................................................................................................. 189 8.2.1 PROTECTION ...................................................................................................................... 189 8.2.2 INTERVENTION .............................................................................................................. 190 8.2.3 MONITORING ................................................................................................................ 191 9.0 References ........................................................................................................... 195 10.0 Appendices .......................................................................................................... 207 7 Executive Summary In 2004, following formal complaints (2003/5272 & 2004/4112) to the European Commission, the UK government became exposed to a very serious risk of infraction for alleged breaches of Articles 2 and 6 of the Habitats Directive. At that time government stated that ‘appropriate regulatory action under Article 6.2 of the Habitats Directive has been undertaken in an attempt to prevent deterioration of the Modiolus reefs’ and undertook ‘to comply with obligation in Article 2.2 of the directive to restore the Modiolus reefs to favourable conservation status.’ The Strangford Lough Management Scheme 2005-2010 (Environment and Heritage Service 2001) identified actions required by the competent authorities, the Departments of the Environment (DOE) and Agriculture and Rural Development (DARD), to address the restoration of Modiolus Biogenic Reefs in Strangford Lough. Consequently, DOE and DARD approved The Strangford Lough M. modiolus biogenic reef restoration plan (MRP) (Anon 2005). Short-term objectives of the MRP were: to introduce total protection for the remaining reefs and to assess whether conditions in appropriate areas are favourable for restoration using pilot translocation experiments. The medium-term objective was to monitor the rate of natural recovery over a five-year period. The plan also aimed to investigate potential intervention techniques that would be needed if natural recovery was not occurring. The ultimate, long-term objective of the plan is ‘to restore the Strangford Lough Modiolus biogenic reef feature to Favourable Conservation Status’ (Anon 2005). To help deliver the plan, DOE and DARD established The Modiolus Restoration Research Group Project in February 2008. Terms of Reference: MRRG delivered technical aspects of the Modiolus biogenic reef restoration plan (MRP) through specific undertakings (Table 1). Carried out over 3 years between February 2008 and February 2011 the project was delivered by three academic, six research and two technical staff. Field surveys involved deployment of 276 Remotely Operated Vehicle (ROV) drops and over 448 dives (Appendices 1 & 2). Laboratory research included preparation for field surveys, analysis of field samples and over 880 days in a hatchery dedicated to the project. An artificial reef experiment was established to investigate the potential for restoration involving translocation of mussels onto cultch. This involved deploying 10 8 tonnes of weathered scallop shells in an experimental array and „seeding‟ this with 6000 Modiolus modiolus broodstock. In addition, a Modiolus researchers‟ conference was hosted by MRRG to initiate networking with specialists in the field (Appendix 3). Table 1. The Modiolus Restoration Research Group Project: Delivering technical aspects of the The Strangford Lough Modiolus biogenic reef restoration plan (MRP). MRP objectives 1. Reef Mapping: to identify, map and introduce total protection for the remaining M. modiolus biogenic reef sites within 1 year of adoption of this plan: damaged biogenic reefs will also be identified and protected from further damage 2. Monitoring natural recovery: to show, using appropriate reference and control sites, evidence of recovery of the M. modiolus biogenic reef feature towards ‘Unfavourable Condition, Recovering’ within 5 years of initiation of this proposed plan 3. Identify suitable sites for restoration: to assess whether conditions in appropriate areas within Strangford Lough are currently favourable for restoration using pilot scale translocation experiments 4. Potential intervention strategies: to restore the Strangford Lough M. modiolus biogenic reef feature to ‘Favourable Conservation Status’ Undertakings of the Modiolus Restoration Research Group Project U1: Map areas of remaining „pristine‟ M. modiolus U2: Identify and map areas of damaged M. modiolus U3: Provide scientific advice for the implementation of restoration and protection of both pristine and damaged areas U4: Monitor temporal trends in „pristine‟ reefs U5: Investigate the potential for natural recovery - damaged reef U6: Investigate the potential for natural recovery - historical range U7: Investigate changes in the distribution of „pristine‟ and damaged M. modiolus reefs U8: Generate projections when „Favourable Conservation Status‟ might be achieved U9: Define conditions favourable for M. modiolus in Strangford Lough U10: Map the suitability of current condition for M. modiolus in Strangford Lough U11: Translocate M. modiolus and monitor recovery U12: Investigate ways to increase provision of suitable substrata for spat settlement U13: Culture M. modiolus for reseeding This section of the report summarises the key findings of the Modiolus Restoration Research Group [MRRG] project and, based on these, provides recommendations whereby the competent authorities (DOE and DARD) might achieve their objectives as stated in the Modiolus Restoration Plan [MRP]. 9 Summary of key findings Changes in the distribution, density and condition of M. modiolus reefs in Strangford Lough (Undertakings 1, 2 and 7) Until the mid 1970s M. modiolus beds were found extensively throughout Strangford Lough. The current distribution, density, and condition of M. modiolus communities in the Lough were assessed and mapped. For the purposes of the present study, „good‟ condition M. modiolus reefs in Strangford Lough were defined as sites with ≥ 5 individuals, and ≥ 1 clump per m -2 and „poor‟ condition M. modiolus reefs were defined as sites with < 5 individuals, and < 1 clump per m -2. M. modiolus beds have declined substantially in distribution, extent, condition and density. Between 1975 and 1995 the distributional extent of M. modiolus in Strangford Lough declined by over 40%. This decline has continued in some areas but at a slower rate. Although densities were not recorded widely or at the same sites over time, density data, where available, reflect the trends seen in distributional data. M. modiolus beds can now be found in an area between Castle Island and Gransha Point in the north and Taggart Island and Kate‟s Pladdy in the south (Figure 1.0). Beds considered in „good‟ condition can be found at Craigyouran and Round Island Pinnacle (Figure 1.0). Remaining beds are fragmented and patchy. Small-scale temporal and spatial variability in ‘good’ and ‘poor’ M. modiolus reefs (Undertakings 4 and 5) This part of the study aimed to monitor short-term trends in „good‟ and „poor‟ condition M. modiolus communities in Strangford Lough to determine whether they are improving or deteriorating over time. Monitoring included in situ counts of M. modiolus, photography of 0.25m2 quadrats and total removal sampling using 10 cm diameter cores. M. modiolus densities were generally higher in the north basin good and poor sites than in south basin good and poor sites respectively and did not change through time. The mean numbers of epifaunal species in photo-quadrats of good and poor sites in the north basin were generally higher than those of good and poor sites respectively in the south basin. Non-metric multidimensional scaling of photo-quadrat 10 data showed that epifauna in the good condition sites in both the north and south basins and in the poor condition site in the north remained relatively stable through time whereas epifauna in the poor condition site in the south basin varied through time. Univariate analysis of core samples revealed no clear temporal trends. However, there were significant differences between the numbers and abundances of species in core-samples from good and poor sites, from the north and south basins, and the different sampling times. Multivariate analysis of core samples suggested little temporal change in communities in good sites in both the north and south basins and in those from poor sites in the north basin. However, communities in the core samples from poor M. modiolus beds in the south showed a distinctive shift in MDS space after 6 months which reflects the significant effects of time and time-related interactions seen in univariate analysis. This part of the study suggests that short-term monitoring is of limited value in following temporal trends because there are significant interactions amongst variables and that future monitoring should be carried out no more frequently than annually to minimise this variability. Potential for natural recovery of M. modiolus reefs (Undertaking 6) In 2003, the M. modiolus communities in Strangford Lough were surveyed by video transects and by quadrat removal sampling in sites north (northern basin) and south (southern basin) of the Long Sheelah (SLECI, Roberts et al., 2004). Sites were selected to sample the historical range of the M. modiolus with Chlamys varia, sponges, hydroids and bryozoans biotope (code SS.SBR.SMus.ModCvar) and the M. modiolus with hydroids and large solitary ascidians (SS.SBR.SMus.ModHAs) biotope, in the north and south basins respectively. To determine the long term potential for natural recovery of impacted M. modiolus communities repeat surveys were carried out at the same sites and using the same methodology as in 2003 , as far as was possible. In the north basin sites there have been further declines in the density and frequency of occurrence of M. modiolus, relative to the 2003 SLECI surveys. In the south basin there have been further declines in the density of M. modiolus, but increases in its frequency of occurrence suggesting greater 11 fragmentation of the biotope. There was a decrease in the mean number of species and Shannon‟s and Pielou‟s diversity indices between 2003 and 2010 in the north basin. There was an increase in the mean number of species but no clear differences in Shannon‟s and Pielou‟s diversity indices between 2003 and 2010 in the south basin. This part of the study suggests that the biotope in the north basin has continued to decline in condition since SLECI, whereas the biotope in the south basin appears to show increased fragmentation although some good condition sites remain. Identifying suitable sites for restoration: habitat suitability modelling for Modiolus modiolus, in Strangford Lough (Undertakings 9 and 10) Species distribution modelling was used to identify suitable habitat for M. modiolus in Strangford Lough to provide objective information on sites where intervention and natural recovery is most likely to be successful. Predictive distribution models were developed for each biotope found within the Lough: the Modiolus, Aequipecten opercularis, and Chlamys varia community (JNCC Biotope code SS.SBR.SMus.ModCvar) found in the Northern basin and the Modiolus, hydroids, ascidians and brittle stars communities (JNCC Biotope codes SS.SBR.SMus.ModT) found in southern basin. M. modiolus presence records were collected during SCUBA dive surveys between 2008 and 2010 and environmental parameters from CEDaR records. Environmental data was interpolated using ARCGIS v9.3 (ESRI, California, USA) to provide environmental layers for distribution modelling using MAXENT, at a common pixel size of 40m. Although substrata importance varied between biotopes, overall M. modiolus distribution was positively associated with the presence of mud and sand and negatively associated with the presence of cobbles, boulders, gravel, bedrock and pebbles. The higher Area under the curve (AUC) value for the Chlamys biotope (SS.SBR.SMus.ModCvar) is most likely an indication of its restricted distribution as opposed to better model fit. Predicted M. modiolus distribution was largely biased towards the centre of the Lough and reflects the known historic distribution. 12 These findings, if combined with larval dispersal modelling, could be used to predict areas of the lough where recovery is more likely and to inform the selection of optimum sites for restoration. Intervention (Undertakings 11, 12 & 13) The Modiolus Restoration Plan requires methodologies to restore the M. modiolus reef feature in the event of natural recovery not being observed. This part of the project used pilot field and laboratory studies to investigate the potential for three intervention techniques: 1) Translocation or restructuring of scattered, un-clumped adult Modiolus modiolus and subsequent monitoring 2) Provision of suitable substrata for spat settlement and subsequent monitoring 3) Pilot Modiolus modiolus hatchery cultivation. Translocation or restructuring of scattered, un-clumped adult Modiolus modiolus and subsequent monitoring An artificial reef was constructed south east of Brown Rocks using weathered King Scallop (Pecten maximus) as cultch for 6000 re-laid adult M. modiolus (Figure 1.0). The experimental design incorporated elevated and flattened artificial reefs. Mussels were also relaid directly on the seabed. The purpose of the experiment was to assess if either cultch or elevation increased survival of the translocated mussels. After 6 months, survival in all treatments was high and differences in mortality rates between treatments were not significant. The number of faunal species associated with the constructed reef increased greatly over six months. This was interpreted as a reflection of the natural reef forming process by M. modiolus. Spat collectors deployed near the cultch site indicate natural recruitment of M. modiolus spat from sources in the surrounding area. Regular monitoring of the artificial M. modiolus reef is required. This should include monitoring the effects of reef elevation and structure on adult mussel survival, natural M. modiolus spat recruitment and diversity of the associated reef community. This experiment has demonstrated that translocation of adult horse mussels on to purposely built artificial reefs consisting of shell cultch is likely to enhance recovery 13 and natural recruitment providing additional brood-stock to damaged areas. The present experiment was conducted within the southern basin of Strangford Lough where the M. modiolus typically belongs to the SS.SBR.SMus.ModHAs/ModT biotope. A similar trial should be conducted at a suitable site in the northern basin to stimulate recovery of the M. modiolus/Chlamys varia (SS.SBR.SMus.ModCvar) biotope. Provision of suitable substrata for spat settlement and subsequent monitoring The Modiolus Restoration Plan proposed the use of suitable substrata to enhance natural recruitment as a potential restoration strategy. The Modiolus Restoration Research Group (MRRG) tested this proposed intervention action by investigating natural recruitment patterns of M. modiolus and spat settlement on different collector materials at several locations representative of its distribution range in Strangford Lough. Natural recruitment was very poor in damaged areas north of the Long Sheelah (ModCvar biotope) but was very high in the southern distribution range (ModHAs biotope), which may be self-sustaining. Settlement rarely occurs outside the matrix created by live adult M. modiolus, and was significantly better among clumps of live mussels than on other materials. Spat settlement was very poor on artificial spat collectors and loose M. modiolus and Pecten maximus shells. The use of artificial blue mussel spat collectors for cultivation of M. modiolus is not a viable restoration approach. The use of seabed cultch techniques to enhance natural recruitment of M. modiolus should be supplemented with translocation of clumps of live M. modiolus. Pilot Modiolus modiolus hatchery cultivation The Modiolus reef Restoration Plan contemplates the production of young M. modiolus for experimental reseeding if natural recruitment is not observed. To meet this objective the Modiolus Restoration Research Group (MRRG) set up the first dedicated M. modiolus hatchery in Europe. Horse mussel spat was successfully produced from local broodstock. The majority of mussels remained ripe throughout the period of study and consequently conditioning was not necessary. Partial 14 desiccation effectively triggered spawning in broodstock mussels. The full larval cycle from fertilized eggs to settled pediveligers takes approximately 38 days at ambient summer water temperatures for Strangford Lough. No significant differences were observed in larval survival and growth using single or mixed algal diets. Pediveligers preferred settling among live mussels than on artificial substrata. Spat up to 1.5mm long were obtained after 4 months in an upwelling system. The main obstacles to producing sufficient quantities of spat for reseeding were: 1) lengthy developmental cycle; 2) slow larval and spat growth; 3) poor survival rates; and 4) very specific settlement requirements. The high costs associated with running the hatchery operations compared to the poor return in seed means hatchery production of M. modiolus is not a viable restoration option at this stage. Projection for recovery of ‘Favourable Conservation Status’ (Undertaking 8) Most of the assessment criteria suggest that the majority of M. modiolus biogenic reefs, particularly biotope SS.SBR.SMus.ModCvar, in Strangford Lough remain in unfavourable conservation status (Table 2). „Good‟ condition sites of biotope SS.SBR.SMus.ModHAs/ModT at e.g. Craigyouran and Round Island Pinnacle are not in pristine condition when compared with other beds in the U.K. However, these probably represent the best remaining M. modiolus communities in the Lough. Because results from short-term temporal monitoring showed no clear trends, it is not possible to develop a model based on the current study to predict when favourable conservation status of M. modiolus biotopes will be restored in Strangford Lough. However, during the course of the current project a dynamic ecosystem carrying capacity model for Strangford Lough was expanded to incorporate additional species including Modiolus modiolus. A model scenario was run, where historical M. modiolus areas were populated at a „pristine‟ density of 50 individuals m-2. The model predicted that M. modiolus in areas close to the mouth of the lough will grow more quickly than those elsewhere and also suggests that food availability is unlikely to be a factor limiting its recovery. In addition, published studies from New Zealand and Canada suggest that impacted bivalve biogenic reefs may take extended periods to recover after the cessation of fishing activities. 15 Factors affecting successional regeneration of bivalve biogenic reefs include the period of non-disturbance, proximity of propagule sources and hydrodynamic influences on propagule dispersal. In Strangford Lough, much of the degraded M. modiolus habitat lies within 10-15 km of sources of propagules from the remaining beds; this suggests that signs of natural recovery might be expected within 20 years in Strangford Lough, provided there is no further disturbance. 16 Table 2. Assessment of the current conservation status of M. modiolus biotopes in Strangford Lough (December 2010) Definitions of ‘Favourable Conservation Status’ Natural Habitat Its natural range is stable or increasing. The specific structure and functions necessary for its long-term maintenance exist and are likely to continue for the foreseeable future. The conservation status of its typical species is favourable. Species (Modiolus modiolus) Population dynamics indicate it is maintaining itself on a long-term basis as part of its natural habitat. Its natural range is not declining or likely to decline in the foreseeable future. There is a sufficiently large habitat to maintain it on a long-term basis. Biotopes SS.SBR.SMus.ModCvar Continuing to decline in distribution and condition. Because there has been continued contraction in the range of its „foundation‟ species, M modiolus, this condition is not met. Although many species recorded in previous surveys are still present and presumably self-maintaining, species diversity has declined between 2003 and 2010. In addition, key species for this biotope (Chlamys varia and Aequipecten opercularis) are missing or underrepresented. SS.SBR.SMus.ModHAs/ModT At some sites this biotope shows increasing fragmentation. „Good‟ condition sites at e.g. Craigyouran and West of Round Island Pinnacle are not in pristine condition when compared with other beds in the U.K. However, these probably represent the best remaining M. modiolus communities in the Lough. This condition is only met at a small number of sites. Most species recorded in previous surveys are still present and presumably selfmaintaining. Species diversity has not declined between 2003 and 2010. Natural recruitment is very poor. Natural recruitment is high. Most of the recent (since 2003) contraction in range has been in the northern basin. Based mainly on substratum characteristics, MAXENT modelling suggests that suitable habitat exists over much of its historical range. Range contraction (since 2003) less evident than in the northern basin. Based mainly on substratum characteristics, MAXENT modelling suggests that suitable habitat exists over much of its historical range. 17 Conclusions Side-scan sonar and video surveys carried out between 1990 and 1993 indicated that areas of Strangford Lough had been heavily impacted by trawling (Service and Magorrian 1997). Recognising the vulnerability of benthic habitats to disturbance by heavy mobile fishing gear the regulatory authorities introduced restrictions to fishing in Strangford Lough in 1993 (Inshore Fishing Prohibition Regulations (NI) 1993). These regulations prohibited the fishing of Modiolus, prohibited the use of tickler chains on fishing gear, restricted scallop dredging to the south of limestone rock, and prevented incursion of mobile gear into the westerly part of the lough. Such management of fishing would give the opportunity to assess recovery of areas impacted by mobile benthic fishing gear (Service and Magorrian 1997). However, by 2003, there was no evidence to suggest recovery of M. modiolus communities in Strangford Lough despite legislation to manage fishing (Roberts 2003). Consequently, DARD extended the prohibition on mobile gear to the whole of Strangford lough in December 2003. The present study has shown that the decline has not been halted by previous management intervention methods and M. modiolus beds in Strangford Lough remain much reduced in extent, density, and condition. Specifically, M. modiolus communities at Black Rocks, Long Sheelah and Selk Rock beds remain in poor condition; those at Craigyouran and Round Island Pinnacle, although not in pristine condition when compared with other U.K. beds, probably represent the best remaining M. modiolus communities in the Lough. Remaining M. modiolus communities in Strangford Lough still face a number of threats which could limit or prevent their recovery. These include increases in the temperature due to global climate change, eutrophication through agricultural inputs, disease and an increase in the intensity of pot fishing. Further restrictions to all fishing methods in the areas were introduced in March 2011 (The Strangford Lough [Sea Fisheries Exclusion Zones] Regulations [Northern Ireland] 2011 No. 360) (Figure 1.0). Because M. modiolus is a long-lived species which supports diverse benthic communities, long-term protection and intervention are required where such communities have been impacted. To establish the effectiveness of intervention measures it is essential that the distribution, condition and density M. modiolus in Strangford Lough are monitored into the future. 18 While Table 2 may appear to paint a bleak picture for the potential recovery of the M. modiolus biogenic reef feature in Strangford Lough, experience in New Zealand (Cranfield et al. 2004) suggests that biogenic reefs comprising Tiostrea (Ostrea) chilensis and Modiolus areolatus may recover over long periods if undisturbed. In addition, a number of positive elements have emerged from the current project. First, species richness remains high at a number of sites. Second, there is evidence of low levels of natural recruitment in the north basin and higher levels of recruitment in the south basin. Third, intervention involving translocation of M. modiolus onto cultch shows a great potential to kick start the regeneration process. These positive elements of the study form the basis of the recommendations below. 19 Recommendations (Undertaking 3) Recommendations below follow the three essential elements of the DARD/DOE Modiolus Restoration plan. PROTECTION Maintain the ban on the use of mobile fishing gear MRRG recommend that a totalprotection zone is established below the 10m contour line between: Castle Island to Gransha Point in the North and the Southern tip of Island Taggart to Kate‟s Pladdy in the South (Fig 1.0). Rationale: 1. The recommendation meets the first objective of the the Modiolus Restoration Plan agreed by DOE and DARD: „to identify, map and introduce total protection for the remaining Modiolus biogenic reef sites within one year of the adoption of this plan; damaged biogenic reefs will also be identified and protected from further damage‟. 2. The proposed total-protection zone: Contains the bulk of remaining M. modiolus communities Contains the experimental restoration cultch site Includes a significant proportion of habitat suitable for M. modiolus biotopes (ModCvar and ModHAs/ModT) based on habitat suitability models Issues: Impacts on current stakeholder activities This issue is without the scope of this report and fall within the responsibilities of the competent authorities, DOE and DARD 20 INTERVENTION Establish at least one new artificial reef within the proposed total-protection zone using weathered cultch. The reef site(s) should be selected on the basis of habitat suitability and larval dispersal modelling, and be located within the historical distribution of the Modiolus modiolus beds with Chlamys varia, sponges, hydroids and bryozoans (SS.SBR.SMus.ModCvar) biotope. Ideally large numbers of adult mussels should be translocated onto cultch in the area(s) selected for translocation. Based on preliminary results from the current study, which found no significant difference in survival of mussels translocated on to elevated or flattened cultch or onto unmodified substrate the use of cultch may be redundant. However, sourcing and deployment of cultch should be budgeted into any further intervention efforts. In addition, because sourcing mussels for translocation remains a problem (see below) the best approach might be to concentrate existing mussels into larger patches. This would stabilise mussel patches due to the clumping behaviour of mussels and overcome Allee effects whereby reproductive success decreases with population density. Rationale: Experimental trials in current project show that: Artificial reefs stabilise quickly Translocated mussels show high survival Reefs are rapidly colonised by epifauna Issues: The key issue involving translocation of mussels is that of acquiring sufficient quantities of mussels to increase the chances of success. This would necessitate collecting mussels from outside Strangford Lough for this purpose. This was considered when the pilot reef experiment was initiated during the present project. The proposal was rejected on the grounds that 1) M. modiolus beds in the western Irish sea (the nearest source stocks) are themselves already under threat (Goodwin et al. 2011); 2) there would be a risk of 21 introducing pathogens and alien species; 3) introduced mussels might not be genetically compatible with populations in Strangford Lough (see for example Maggs 2008). However, because this project has successfully translocated mussels in small-scale trials, the potential risks and benefits of such intervention should be re-evaluated with a view to undertaking translocation on a large scale. Cost MONITORING Establish an annual programme to monitor: status of natural biogenic reefs recruitment & succession on established experimental reef recruitment & succession on proposed experimental reef selected historical sites where M. modiolus no longer occurs Rationale: Longer time frame is required to demonstrate: positive or negative changes in natural reefs (natural recovery) effectiveness of artificial reefs Issues: Cost 22 Figure 1.0 Distribution of Modiolus modiolus in Strangford Lough in 2010 based on surveys conducted by the Modiolus Restoration Research Group (Inset). M. modiolus reefs were recorded at 123 of 442 sites surveyed and is the basis of the recommendation to establish a total protection zone below the 10m depth contour, between Castle Island to Gransha Point in the North and the Southern tip of Island Taggart to Kate‟s Pladdy in the South (Main figure). The recommendation aims to meet the first objective of the Modiolus Restoration Plan agreed by DOE and DARD: „to identify, map and introduce total protection for the remaining Modiolus biogenic reef sites within 1 year of the adoption of this plan‟. The map also indicates: The position of „good‟ condition sites, at Craigyouran and Round Island Pinnacle The experimental cultch site Strangford Lough (Sea Fisheries Exclusion Zones) Regulations (Northern Ireland) 2011 No. 36, Introduced 14th March 2011 are also illustrated. 23 STRANGFORD LOUGH ARDS PENINSULA IRISH SEA Proposed M. modiolus protected area Sea Fisheries Exclusion Zones March 2011 Experimental M. modiolus reef site MRRG survey records (2008-2010) M. modiolus present M. modiolus absent 24 1.0 Introduction 1.1 The Modiolus Restoration Research Project This report describes the findings of the Modiolus Restoration Research Group [MRRG] Project set up to deliver technical aspects of the MRP through specific undertakings (Table 1.1) and to recommend a strategy whereby the competent authorities might achieve these objectives. Carried out over 3 years between February 2008 and February 2011 the project was delivered by three academic, six research and two technical staff. Field surveys involved deployment of 276 Remotely Operated Vehicle (ROV) drops and over 448 dives (896 hours, each diver clocking over 112 hours of diving bottom time) (Appendices 1 & 2). Laboratory research included preparation for field surveys, analysis of field samples and over 880 days in a hatchery dedicated to the project. An artificial reef experiment was established to investigate the potential for restoration involving translocation of mussels onto cultch. This involved deploying 10 tonnes of weathered scallop shells in an experimental array and „seeding‟ this with 6000 Modiolus modiolus broodstock. In addition, a Modiolus researchers‟ conference was hosted by MRRG to initiate networking with specialists in the field (Appendix 3). 25 Table 1.1 The Modiolus Restoration Research Group Project: Delivering technical aspects of the The Strangford Lough Modiolus biogenic reef restoration plan (MRP) MRP objectives 1. Reef Mapping: to identify, map and introduce total protection for the remaining M. modiolus biogenic reef sites within 1 year of adoption of this plan: damaged biogenic reefs will also be identified and protected from further damage 2. Monitoring natural recovery: to show, using appropriate reference and control sites, evidence of recovery of the M. modiolus biogenic reef feature towards ‘Unfavourable Condition, Recovering’ within 5 years of initiation of this proposed plan 3. Identify suitable sites for restoration: to assess whether conditions in appropriate areas within Strangford Lough are currently favourable for restoration using pilot scale translocation experiments 4. Potential intervention strategies: to restore the Strangford Lough M. modiolus biogenic reef feature to ‘Favourable Conservation Status’ Undertakings of the Modiolus Restoration Research Group Project U1: Map areas of remaining „pristine‟ M. modiolus U2: Identify and map areas of damaged M. modiolus U3: Provide scientific advice for the implementation of restoration and protection of both pristine and damaged areas U4: Monitor temporal trends in „pristine‟ reefs U5: Investigate the potential for natural recovery - damaged reef U6: Investigate the potential for natural recovery - historical range U7: Investigate changes in the distribution of „pristine‟ and damaged M. modiolus reefs U8: Generate projections when „Favourable Conservation Status‟ might be achieved U9: Define conditions favourable for M. modiolus in Strangford Lough U10: Map the suitability of current condition for M. modiolus in Strangford Lough U11: Translocate M. modiolus and monitor recovery U12: Investigate ways to increase provision of suitable substrata for spat settlement U13: Culture M. modiolus for reseeding As information and experience developed during the course of the project it was necessary to make slight changes to the methodology proposed in the tender to deliver undertakings listed above; these are documented in the relevant sections below. Some minor regrouping of the undertakings has also been made to improve clarity. 26 The report provides the rationale and objectives of the project followed by a series of technical reports detailing the main findings: 1. Introduction: Background; Rationale; Objectives (this Section) TECHNICAL REPORTS 2. Changes in the distribution, density and condition of M. modiolus reefs in Strangford Lough (Undertakings 1, 2 and 7) 3. Temporal trends in M. modiolus communities in Strangford Lough (Undertakings 4 & 5) 4. Potential for natural recovery of M. modiolus communities in Strangford Lough (Undertaking 6) 5. Identifying suitable sites for restoration: habitat suitability modelling for M. modiolus in Strangford Lough (Undertakings 9 & 10) 6. Intervention (Undertakings 11, 12 & 13) 7. Projection for recovery of „Favourable Conservation status‟ (Undertaking 8) 8. Recommendations (Undertaking 3) 27 1.2 Background 1.2.1 Strangford Lough Covering over 15000 ha Strangford Lough is the largest sea inlet in Britain and Ireland. It has a narrow inlet from the Irish Sea which has resulted in a unique hydrography. This unique hydrography, together with its glacial origin, has created a wide range of habitats supporting a high diversity of marine communities with more than 2000 recorded species (Environment and Heritage Service 2001). Strangford Lough was designated as Northern Ireland's first Marine Nature Reserve and has been identified as a pilot Marine Protected Area (MPA) (Cork 2006). It is listed as a NATURA 2000 area [UK0016618] (JNCC 2011). A key feature in this designation is the presence of biogenic reefs including Modiolus modiolus reefs. 1.2.2 The importance of bivalve biogenic reefs Complex habitats in the marine environment, such as sea-grass beds, coral reefs and other invertebrate biogenic reefs support high biodiversity (Cranfield et al. 2004; Holt et al. 1998), which provide important ecosystem services. Oysters and mussels in particular provide a wide range of ecological services such as seston filtration, benthic-pelagic coupling, feeding and structural habitat for mobile species as well as hard surfaces for attachment of sessile species (Coen et al. 2007) and stabilising the seabed (Jones 1952; Rees 2009). When such habitats are compromised these features and services are reduced or lost and may either recover slowly or not at all without intervention. Biogenic reefs formed by bivalves such as oysters are one of the most endangered habitats on earth; on a global scale it is estimated that up to 85% of oyster reefs have been lost since the 19th century as a result of overfishing and coastal degradation and many may be functionally extinct (Beck et al. 2009). Most studies on impacted oyster reefs to date, including those on habitat restoration (Cranfield et al. 2004; Trimble et al. 2009) have focused on commercial aspects. However, there is an increasing awareness that it is essential to take an ecosystem approach to ensure that fisheries are managed sustainably in a way that protects 28 habitats and conserves biodiversity in the marine environment (Beck et al. 2009; Palumbi et al. 2009). Mussels, the second major reef-building bivalve taxon after oysters, support high biodiversity because they increase habitat complexity (Koivisto and Westerbom 2010). Species such as Mytilus californianus can form biogenic structures up to 40cm deep (Seed & Suchanek 1992); the horse mussel (Modiolus modiolus) can develop a complex undulating topography with amplitudes over 1m in the UK (Rees et al. 2008) and up to 3m in the Bay of Fundy (Wildish et al. 1998). In addition, complex biogenic reefs including both oysters (Tiostrea (=Ostrea) chilensis) and mussels (Modiolus areolatus) have been reported from conditions of strong tidal currents in the Foveaux Strait, New Zealand (Cranfield et al. 2004 and references therein). The horse mussel (Figure 1.1) is a widely distributed boreal species, which can grow up to 200mm and may live up to 100 years old (Anwar et al. 1990). M. modiolus communities occur from the low intertidal (Davenport & Kjørsvik 1982) to over 100m depth (Tendel & Dinesen 2005). Mair et al. (2000) categorized literature dealing with different aspects of the biology of M. modiolus. M. modiolus can be regarded as a „keystone‟ or „foundation‟ species for four habitat types currently recognized in Europe (Connor et al. 2004; Rees 2009; EUNIS 2011): 1. Modiolus modiolus beds with hydroids and red seaweeds on tide-swept circalittoral mixed substrata (EUNIS Code: A5.621; JNCC 04.05 code: SS.SBR.SMus.ModT) 2. Modiolus modiolus beds on open coast circalittoral mixed sediment (EUNIS Code:A5.622; JNCC 04.05 code: SS.SBR.SMus.ModMx) 3. Modiolus modiolus beds with fine hydroids and large solitary ascidians on very sheltered circalittoral mixed substrata (EUNIS Code: A5.623; JNCC 04.05 code: SS.SBR.SMus.ModHAs) 4. Modiolus modiolus beds with Chlamys varia, sponges, hydroids and bryozoans on slightly tide-swept very sheltered circalittoral mixed substrata (EUNIS Code: A5.624; JNCC 04.05 code: SS.SBR.SMus.ModCvar) 29 Figure 1.1 The horse mussel, Modiolus modiolus. 30 1.3 Rationale Modiolus modiolus has been documented in Strangford Lough since the midnineteenth century and was known to form widely distributed beds or reefs in the Lough in the 1970s. The first attempts to map the full extent of these beds were conducted in the 1990s by which time it was evident that some of the beds had already been significantly impacted by trawling (Service and Magorrian 1997). Up to three of the known M. modiolus biotopes occur in Strangford Lough (Roberts et al. 2004): EUNIS Codes A5.621; A5.623; A5.624. One of these habitat types (A5.624) was included, because of its relative rareness, as one of the key features listed as a selection feature under Annex 1 of the Habitats Directive in the designation of the Lough in 2002 as a Special Area of Conservation (SAC) (JNCC 2011). EUNIS habitat type A5.624 occurs on mixed substrata in very sheltered slightly tide-swept conditions and is characterised by Chlamys varia, sponges, hydroids and bryozoans on M. modiolus beds with (JNCC 04.05 biotope code: SS.SBR.SMus.ModCvar). Following concerns raised by various Non-Governmental Organisations (NGOs) and others that the designated feature (A5.624) was not in favourable conservation status, the Department of the Environment (DoE) commissioned the Strangford Lough Ecological Change Investigation (SLECI) in 2003 to investigate ecological changes in the Lough. A major element of this broader study was to investigate both the current status of M. modiolus biogenic reefs and identify probable causes of their decline. SLECI reported a severe decline in the reefs with reference to surveys carried out in the 1970s and 1980s and confirmed that the reefs were not in favourable conservation status (Roberts et al. 2004). An interim report by SLECI in November 2003 identified trawling as the single most likely cause of reef decline. As a result, the Department of Agriculture and Rural Development (DARD) introduced a total ban on all mobile fishing gear within Strangford Lough in December 2003. This ban is still in place. In 2004, following formal complaints (2003/5272 & 2004/4112) to the European Commission, the UK government became exposed to a very serious risk of infraction 31 for breaches of Articles 2 and 6 of the Habitats Directive. At that time government stated that ‘appropriate regulatory action under Article 6.2 of the Habitats Directive has been undertaken in an attempt to prevent deterioration of the Modiolus reefs’ and undertook ‘to comply with obligation in Article 2.2 of the directive to restore the Modiolus reefs to favourable conservation status’. The Strangford Lough Management Scheme 2005-2010 (Environment and Heritage Service 2001) identified actions required by the competent authorities, the Departments of the Environment (DOE) and Agriculture and Rural Development (DARD), to address the restoration of Modiolus Biogenic Reefs in Strangford Lough. Consequently, DOE and DARD approved The Strangford Lough M. modiolus biogenic reef restoration plan (MRP) (Anon 2005). The purpose of the MRP is to establish mechanisms whereby the Competent Authorities within Northern Ireland might attempt to restore Strangford Lough Special Area of Conservation (SAC) to Favourable Conservation Status (FCS). The MRP aimed to ensure maximum protection for the remaining reefs and sites where M. modiolus had recently been recorded, while monitoring the rate of natural recovery over a five-year period. The plan also aimed to pilot potential intervention techniques that would be needed if natural recovery was not occurring. The plan identifies three prime objectives: “The short term objectives are: to identify, map and introduce total protection for the remaining M. modiolus biogenic reef sites within 1 year of adoption of this plan: damaged biogenic reefs will also be identified and protected from further damage to assess whether conditions in appropriate areas within Strangford Lough are currently favourable for restoration using pilot scale translocation experiments The medium term objective is: 32 to show, using appropriate reference and control sites, evidence of recovery of the M. modiolus biogenic reef feature towards „Unfavourable Condition, Recovering‟ within 5 years of initiation of this proposed plan. The long-term objective is: to restore the Strangford Lough M. modiolus biogenic reef feature to „Favourable Conservation Status‟.” 33 TECHNICAL REPORTS 34 2.0 Changes in the distribution, density and condition of Modiolus modiolus communities in Strangford Lough (Undertakings 1, 2 and 7) 2.1 Summary Until the mid 1970s M. modiolus was found extensively throughout Strangford Lough. This study aimed to identify areas of M. modiolus beds and assess the changes in their distribution, density, and condition in Strangford Lough since the 1970s. The current survey found that M. modiolus beds have declined substantially in distribution, extent, condition and density. M. modiolus beds can now be found in an area between Castle Island to Gransha Point in the north and Island Taggart to Kate‟s Pladdy in the south. Beds considered in „good‟ condition can be found at Craigyouran and west of Round Island Pinnacle; however, in other areas the beds are fragmented and patchy. Between 1975 and 1995 the distributional extent of M. modiolus in Strangford Lough declined by over 40%. This decline has continued in some areas but at a slower rate. Although densities were not recorded widely or at the same sites over time, density data, where available, reflect the trends seen in distributional data. The present data suggest that at the current rate of decline, M. modiolus could be become regionally extinct in Strangford Lough without intervention. 35 2.2 Introduction M. modiolus is a long lived marine bivalve found in a wide range of habitats and locations throughout the UK (Rees 2009). Although M. modiolus is a widespread and common species, horse mussel beds (areas with ≥ 30 % of mussel m-2) are only found in a limited number of locations (Rees 2009). M. modiolus beds consist of the mussels themselves and the accumulation of shell and faecal deposits (Sanderson et al. 2008; Rees et al. 2008). Mapping and monitoring the distribution and densities of M. modiolus beds is crucial for drawing conclusions about their population trends and conservation status. Previous efforts to map the distribution and monitor the densities of M. modiolus in Strangford Lough have included diver-based surveys (Roberts 1975; Erwin et al. 1986; Brown 1989; Erwin et al. 1990;; Roberts et al. 2004), and surveys involving remotely operated vehicles (Service 1990; Service 1998) and Acoustic Ground Discriminate Systems (AGDS) (Service and Magorrian 1997; Magorrian and Service 1998; Magorrian et al. 1995; Mitchell & Service 2003; Roberts et al. 2004). Where possible these surveys were used to determine the historical distribution and condition of M. modiolus communities. The aim of this part of the project was to document temporal trends in the extent and condition of M. modiolus communities in Strangford Lough. Specific objectives were to: Map areas of remaining good M. modiolus communities. (Undertaking 1) Identify areas of poor M. modiolus communities. (Undertaking 2) Identify changes in the distribution of good and poor M. modiolus communities. (Undertaking 7) 36 2.3 Methods 2.3.1 Remotely Operated Vehicle surveys (2008-2010) The broad-scale mapping of the distribution of live and dead M. modiolus in Strangford Lough involved the use of a Remotely Operated Vehicle (ROV; VideoRay®) (Figure 2.1), which was deployed at 276 sites between 2008 to 2010 (Appendix 1). Figure 2.1 VideoRay® Remotely Operated Vehicle. Sites surveyed were selected using a grid baseline overlaid on admiralty chart data for Strangford Lough. This survey was extended to include a higher resolution investigation of the seabed within and around current and historical M. modiolus beds at five sites only (Long Sheelah, Hadd Rock, Black Rocks, Round Island Pinnacle and Craigyouran). At each survey site, the ROV was flown for 5 minutes on a weighted tether 9 m long, in a circle (approximate circumference 55 m). The presence, condition and clump size of live and dead M. modiolus, associated epifauna and substratum type 37 was recorded in the field. In the laboratory, the footage was carefully inspected by two members of staff to augment the field notes. Because the ROV has a very limited field of view and operators are unable to examine mussels in detail, dives were conducted at 105 sites to ground truth these data (see section 2.3.2 below for details). 2.3.2 Dive surveys (2008-2010) To ground truth the ROV surveys, dives were conducted at 105 sites, selected randomly to cover a wide range of sites in both the north and south basins (Appendix 2). Divers recorded the presence, condition (section 2.3.5.1) and clump size of live and dead M. modiolus at the beginning and end of each dive. To monitor the densities of M. modiolus through time the divers collected all mussels from replicated 0.25 x 0.25 m quadrats, at four sites. These sites Bird Island Passage (n = 3), Brown Rocks (n = 8), Long Sheelah (n = 12), and west of Round Island Pinnacle (n = 8), (fig. 2.2) were chosen based on historical samples (Roberts 1975; Erwin et al. 1990; Roberts et al. 2004). 38 Figure 2.2 Location of the 0.25 x 0.25 m removal quadrat sites in the historical sites (Bird Island Passage, Black Rocks, Long Sheelah and Round Island Pinnacle) in Strangford Lough. 2.3.3 Ultra Short Baseline acoustic surveys (2008-2010) To map the boundary of the M. modiolus communities, two divers equipped with SCOUT® USBL (Ultra Short Baseline) acoustic referencing systems (Sonardyne Ltd.) swam the edges of M. modiolus communities, using DPV (Diver Propulsion Vehicles). The USBL system provides an accurate positioning for the divers, which when integrated with a dGPS string, compass/transducer heading and PC can be imported into ArcGIS. Two divers entered the water at a designated USBL point and travelled using DPVs until a recognisable M. modiolus bed edge was found. The divers advised topside on commencement of the bed mapping. The divers travelled the bed edge using compass directions to maintain a zig-zag search pattern (Figure 2.3). The zig-zag 39 pattern means that divers are actually doing oblique transects across the boundary, hence providing the divers absolute certainty that the boundary has been found. A 5m length was set for each leg of the zig-zag pattern. The divers advised top-side each time the bed edge was passed during the travel. Top-side plotted a way-point on the advice of the divers. USBL plotted way-points Divers path of travel M. modiolus bed edge Figure 2.3 Search pattern followed by divers during the DPV mapping of M. modiolus bed boundaries. Following the boundaries of clumped M. modiolus proved to be too difficult. It was apparent that clumped and damaged M. modiolus were not at all distinct but actually formed very subtle gradients between fairly small areas of clumped areas, and this methodology was deemed to be unsuitable and not used after these scoping attempts were carried out in 2008. 2.3.4 Historical records (1954-2007) In order to judge the change in the extent of and damage to M. modiolus communities, it was important to document as accurately as possible all historic records of M. modiolus and its associated assemblages. All historical records were collated from those held by the Centre for Environmental Data Recording (CEDaR), the National Biodiversity Network Gateway (NBNC), Northern Ireland Agri-Food and Biosciences Institute (AFBI), Northern Ireland Environment Agency (NIEA), the Ulster Museum and other published literature. These data were imported into a georeferenced database in ArcGIS. Data included: The dive surveys conducted by Roberts (1975) 40 The dive surveys reported in Seed & Brown (1977; 1978) The dive surveys (1973 to 1977) conducted by the Ulster Museum (Erwin 1986) The Northern Ireland Sublittoral Survey (NISS; 1982-1985) conducted by the Ulster Museum (Erwin et al. 1990) The dive and grabs surveys (1989-2007) conducted by the Ulster Museum (Brown 1989, Nunn 1994) The ROV, grabs and video surveys (1990 to 2003) conducted by AFBI (Service 1990, Magorrian 1995, Magorrian & Service 1998) The dive and grabs surveys (1997 onwards) conducted by NIEA The Strangford Lough Ecological Change Investigation dive and video surveys conducted by Queens University, AFBI, and the Ulster Museum (Roberts et al. 2004) The Sublittoral Survey of Northern Ireland dive surveys (2007) conducted by the Ulster Museum and NIEA The dredging surveys by Williams (1954) and AGDS surveys by Magorrian et al. (1995), Mitchell & Service (2003) and Roberts et al. (2004) were also collected for the geo-referenced GIS database. However, these records were not used in the subsequent analyses because of methodological difficulties in identifying M. modiolus beds using the AGDS (Magorrian & Service 1998; Mitchell & Service 2003; Roberts et al. 2004) and the limited number records in the dredging surveys (Williams 1954). 41 2.3.5 Analyses 2.3.5.1 Distribution and condition of M. modiolus communities All historical and current surveys of Strangford Lough were converted to presence/absence records of live M. modiolus, percentage cover of dead M. modiolus and condition of M. modiolus communities, and biotope types. The condition of M. modiolus communities was defined using dive descriptions and SACFOR categories (as per JNCC website) for historical data and counts for the current project (Table 2.1). The percentage cover of dead M. modiolus was also estimated for the current project (Table 2.2). The historical and current presences/absence records of live M. modiolus and condition of M. modiolus communities, the biotope types and the current percentage cover of dead M. modiolus were then plotted onto maps using ARCGIS v9.2 (ESRI, California, USA). Table 2.1 Definitions used to assign conditions to live M. modiolus communities using historical and current surveys. These colour codes are depicted on figure 2.5 M. modiolus condition Grade Continuous clumps (> 5 Ind. clump-1) or > 1 clump m-2, super abundant, abundant, dense, continuous, bed, thick, frequent 1 Discrete clumps (> 3 Ind. clump-1) < 1 clump m-2 , frequent, occasional, patchy, damaged, clumps 2 Present ( 0 – 3 Ind. clump -1) < 1 clump m -2, rare, present 3 Absent 4 42 Table 2.2 Definitions used to assign categories to percentage cover of dead M. modiolus in the current surveys. These colour codes are depicted on figure 2.6. Categories of dead M. modiolus Continuous coverage (> 70%) 1 40 - 70% 2 20 - 40% 3 0 - 20% 4 2.3.5.2 Projected trends in M. modiolus populations Historical surveys were largely based on different sampling scales and replication and thus cannot be used definitively to establish the distribution and extent of M. modiolus in Strangford Lough at a single point in time. Therefore, to „hindcast‟ its likely historical distribution all records, including current records of M. modiolus were added cumulatively to the earliest records of the species. The condition of M. modiolus communities was similarly „hindcast‟ based on contemporary descriptions to generate Figure 2.5a below. This methodology adopts the approach used by Wilson and Roberts (2011) to establish historical distribution patterns of Margaritifera margaritifera in the UK and Ireland, and is based on the assumption that M. modiolus is a long lived sessile species that is highly unlikely to colonise a new site in a short period of time. In addition, the survey effort for M. modiolus in Strangford Lough has increased through time, and any apparent decline in this species is unlikely to be caused by under recording. The number of 0.5 km grid squares occupied by M. modiolus was then plotted against time. In addition to the distributional data, changes in the density of M. modiolus through time from 0.25 x 0.25 m removal quadrats were also plotted. These data were obtained from four sites in Strangford Lough (Bird Island Passage, Black 43 Rocks, Long Sheelah, and Round Island Pinnacle) from surveys between 1975 and 2010. 44 2.4 Results 2.4.1 Distribution of M. modiolus Historically, M. modiolus was found extensively throughout Strangford Lough (Figure 2.4a). The present study (2008-2010) shows that the distribution of M. modiolus in the Lough is much less extensive than it was previously (Figures 2.4b & 2.7). M. modiolus beds with C. varia, sponges, hydroids and bryozoans (Biotope code SS.SBR.SMus.ModCvar) historically recorded from the northern part of the Lough (Figure 2.5c), are now reduced to a very small area around Long Sheelah. M. modiolus beds with hydroids and large solitary ascidians (Biotope code SS.SBR.SMus.ModHAs) and M. modiolus beds with hydroids and red seaweeds on (Biotope code SS.SBR.SMus.ModT) historically recorded from the southern part of the Lough (Figure 2.5c) are now reduced to four main areas: Black/Brown rocks, Craigyouran, Selk Rocks and Round Island Pinnacle. 45 Figure 2.4a The historic distribution of M. modiolus in Strangford Lough, Northern Ireland. Open circles represent negative records, solid circles represent positive records. 46 Figure 2.4b The current (2008-2010) distribution of M. modiolus in Strangford Lough, Northern Ireland. Open circles represent negative records, solid circles represent positive records. 47 Figure 2.4c. Projected hindcast of the historical distribution of M. modiolus biotopes in Strangford Lough based on data sources listed in section 2.3.4. 48 2.4.2 Condition of M. modiolus communities Historically, „good‟ condition M. modiolus communities, consisting of continuous clumps, were found widely in Strangford Lough (Figure 2.5a). The current survey found that such good condition communities are now restricted to two remaining areas, Craigyouran and west of Round Island Pinnacle (Figure 2.5b). The M. modiolus communities at Long Sheelah, Black Rocks and Selk Rock are no longer in good condition (category 2, Table 2.1). The remaining M. modiolus communities in other areas have become increasingly fragmented or have disappeared (Figures 2.5a,b). 49 Figure 2.5a Historic (1975-2007) condition of M. modiolus communities in Strangford Lough. Each colour code represents the condition of M. modiolus communities, where 1) red = continuous clumps, 2) orange = discrete clumps, 3) yellow = present and 4) green = absent. 50 Figure 2.5b The current (2008-2010) condition of M. modiolus communities in Strangford Lough, Northern Ireland. Each colour code represents the condition of M. modiolus communities, where 1) red = continuous clumps, 2) orange = discrete clumps, 3) yellow = present and 4) green = absent. 51 2.3.3 Percentage cover of dead M. modiolus The current survey found that dead M. modiolus can be found extensively throughout Strangford Lough (Figure 2.6). In both the historic and current sites the cover of dead M. modiolus ranged between 20-70%.The cover of dead M. modiolus was highest in sites with living M. modiolus in the centre of the Lough and lowest in the historical sites (Figure 2.6). The presence of dead M. modiolus can be used to indicate its historical range and potential sites for restoration. 52 Figure 2.6 The current (2008-2010) percentage of dead M. modiolus in Strangford Lough, Northern Ireland. Each condition code represents the percentage cover of dead M. modiolus, where 1) red = > 70% cover, 2) orange = 40-70% cover, 3) yellow 20-40% cover, and 4) green = 0-20% cover. 53 2.3.4 Historical trends in M. modiolus distribution and densities in Strangford Lough The distribution (based on occurrence in 0.5 km grid squares) and the densities of M. modiolus (mussel m-2 at selected sites) in Strangford Lough have substantially declined through time (Figures 2.4, 2.7 & 2.8). There appears to have been a major decline in distribution in the mid 1970s followed by a continuing gradual decline between 1975 and 1995. Between 1975 and 1995 the distributional extent of M. modiolus in Strangford Lough declined by over 40%. Distributional records showed little change between 1995 and 2003 and a slight decline between 2003 and 2010. Although densities were not recorded at the same sites over time, density data were available for four sites at different dates (Figure 2.8). Densities at these sites declined substantially between 1975 and 2003 and further until 2010 (Figure 2.8). Figure 2.7 Rate of decline of M. modiolus distribution through time. Historical trends were derived by hindcasting each data set to the previous earlier records showing a wider distribution. This approach is based on the assumption that a record at a site at any time forms part of its historical range (Wilson and Roberts 2011) and generates the plateaus seen in the plot. Diamonds represent the number of 0.5 km grid squares occupied by M. modiolus. 54 Figure 2.8 The changes in the mean (± SE) number of M. modiolus from 1970 to 2010 (years) at four sites (Bird Island Passage, Black Rocks, Long Sheelah and West Round Island Passage) in Strangford Lough. The number of quadrats varied through time (1975 n = 17, 2003 n = 26, 2010 n = 30). 2.4 Discussion M. modiolus beds in Strangford Lough are well documented (Seed & Brown 1977; Brown 1984; Erwin et al. 1990; Magorrian & Service 1998; Roberts et al. 2004). In the mid 1970s M. modiolus beds were found extensively throughout Strangford Lough (Erwin 1970; Erwin et al. 1986; Erwin et al. 1990). These beds were recorded at depths between 10 and 40 m, with an average number of 134 individuals per m -2, and were either continuous (50% cover m -2) or aggregated into discrete clumps (3-6 m-2). The current research confirms previous reports (Roberts et al. 2004) that the M. modiolus communities have declined extensively in distribution, density and condition in Strangford Lough. Over 40 % of the decline occurred between 1975 and 1995, possibly as a result of trawling impact (Service and Magorrian 1997). Current populations of M. modiolus are largely confined to the centre of Strangford Lough. 55 The average number of M. modiolus has now declined to 3.8 individuals per m 2, which aggregate to form 1-2 discrete clumps m-2. The present data suggest that at the current rate of decline, this species could become regionally extinct in Strangford Lough without intervention. M. modiolus beds can be divided into several community types or biotopes (Connor et al. 2004). At least three of these biotopes are found in Strangford Lough. The current research suggests that the M. modiolus beds in the Northern basin (SS.SBR.SMus.ModCvar biotope) are now confined to the Long Sheelah area, and are no longer in good condition. Beds of the M. modiolus biotopes SS.SBR.SMus.ModHAs and SS.SBR.SMus.ModT in the Southern basin are reduced to four main areas: Black Rock, Craigyouran, Selk Rock, and Round Island Pinnacle. The Black Rock and Selk Rock areas are also not in good condition. Surveys carried out by MRRG suggest that the M. modiolus beds in Round Island Pinnacle and Craigyouran, although not in pristine condition when compared with other U.K. beds, probably represent the best remaining M. modiolus communities in the Lough. M. modiolus has been defined as a key indicator species in a number of long term studies on the impacts of mobile fisheries both in the UK and Canada (Bradshaw et al. 2002; Kenchington et al. 2007). Side scan sonar surveys in Strangford Lough in the 1990s suggested that the M. modiolus beds in the Northern basin were extensively damaged through trawling and dredging for queen scallops (Aequipecten opercularis) (Service and Magorrian 1997; Magorrian & Service 1998). These fisheries commenced in the early 1900s but peaked in activity throughout the mid 1980s to late 1990s (Roberts et al. 2004). Impacts of mobile fishing gear include death or damage of individuals through contact with the gear, or direct removal, fragmentation of clumps resulting in increased risk of predation (Veale et al. 2000; Kenchington et al. 2007). Video surveys were used by Service and Magorrian (1997) to categorise the impact of trawling on M. modiolus communities in Strangford Lough on a scale from 1 (intact M. modiolus communities showing no impact of trawling) to 5 (heavily impacted). Using this method trawling impact was evident in over 80% and 70% of the areas surveyed in 1990 and 1993 respectively (Service and Magorrian 1997). The categories described by Service and Magorrian (1997) were developed further in Magorrian and Service (1998) who reported separation of habitat types using cluster and detrended correspondence analysis. The current study focused on documenting separately the distribution of areas of different categories of live and 56 dead M. modiolus where natural recovery might be predicted in the medium to longer term. Mobile fisheries in Strangford Lough were banned in December 2003. As early as 1997, Service and Magorrian had pointed out that such management of fishing would give the opportunity to assess recovery of areas impacted by mobile benthic fishing gear. The current study found no evidence of recovery of M. modiolus communities. The remaining M. modiolus populations are extensively fragmented in many areas and still face a number of threats which could limit or prevent their recovery. These include increases in the temperature due to global climate change, eutrophication through agricultural inputs, disease and an increase in the intensity of pot fishing. The current research suggests that M. modiolus populations are still declining in some areas and further intervention is required. 2.5 Conclusions The current research has confirmed that M. modiolus beds in Strangford Lough remain much reduced in extent, density, and condition and suggest that the decline has not been halted by previous intervention methods. The present study indicates that the M. modiolus communities at Black Rocks, Long Sheelah and Selk Rock beds remain in poor condition whereas those at Craigyouran and Round Island Pinnacle are in good condition. Because M. modiolus is a long-lived species longterm protection and intervention are required. It is also essential that an annual monitoring programme is established to record the presence, condition and density M. modiolus at predetermined locations. 57 3.0 Small-scale temporal and spatial variability in ‘good’ and ‘poor’ M. modiolus reefs (Undertakings 4 and 5) 3.1 Summary This part of the study aimed to monitor short-term trends in „good‟ and „poor‟ condition Modiolus modiolus communities in Strangford Lough to determine whether they are improving or deteriorating over time. Monitoring included in situ counts of M. modiolus, photography of 0.5 x 0.5m quadrats and total removal sampling using 10 cm diameter cores. Analysis of in situ counts revealed significantly higher densities of M. modiolus in„good‟ relative to „poor‟ condition sites. M. modiolus densities were generally higher in the north basin good and poor sites than in south basin good and poor sites respectively and did not change through time. Analysis of photo-quadrats revealed that, the mean numbers of epifaunal species in the good and poor sites in the north basin were generally higher than good and poor sites respectively in the south basin. Non-metric multidimensional scaling (nMDS) of photo-quadrat data showed that epifauna in the good condition sites in both the north and south basins and in the poor condition site in the north remained relatively stable through time whereas epifauna in the poor condition site in the south basin varied through time. Univariate analysis of core samples revealed no clear temporal trends. However, there were significant differences between the numbers and abundances of species in core-samples from good and poor sites, from the north and south basins, and the different sampling times This part of the study suggests that short-term monitoring is of limited value in following temporal trends because there are significant interaction effects and that future monitoring should be carried out no more frequently than annually to minimise this variability. 58 3.2 Introduction Modiolus modiolus communities are species rich (Holt et al. 1998; Roberts et al. 2004; Rees et al. 2008; Sanderson et al. 2008) and have a limited distribution in the UK (Holt et al. 1998). These communities are comprised of the mussels themselves, the epifauna attached to the mussels, infauna in the interconnecting byssal network, and in biogenic sediments generated by the mussels, vagile epifauna and predators (Roberts 1975; Brown 1989). Because of their high biodiversity (Roberts 1975; Mair et al. 2000; Roberts et al. 2004; Moore et al. 2006; Rees et al. 2008; Sanderson et al. 2008) Modiolus communities have been identified as important features in Special Areas of Conservation and as a priority habitat by the Oslo Paris Commission (OSPAR). However, some M. modiolus beds have been degraded or destroyed by mobile fishing gear (Service & Magorrian 1997; Veale et al. 2001; Roberts et al. 2004). The M. modiolus beds in Strangford Lough, Northern Ireland have a high diversity of associated fauna (>270 taxa) (Roberts 1975; Erwin et al. 1990; Roberts et al. 2004). Three M. modiolus biotopes are found in Strangford Lough (Section 2.4). Surveys in the 1990s and 2000s suggested that M. modiolus beds in Strangford Lough were extensively damaged by mobile fishing gear, particularly in the northern basin (Service & Magorrian 1997). To address this problem the Department of Agriculture and Rural Development of Northern Ireland (DARD) introduced a ban on the use of mobile fishing gear in Strangford Lough in 2004. The aim of this part of the project was to monitor the temporal trends in „good‟ and „poor‟ condition M. modiolus sites (Figure 3.1) in the north and south basins after the introduction of the ban. Specific objectives were to: Monitor the temporal trends in „good‟ condition M. modiolus reefs. (Undertaking 4) Assess the potential for natural recovery within poor condition M. modiolus reefs. (Undertaking 5) 59 A B B C D Figure 3.1 Photographs of (A) north poor condition site (B), north good condition site (C), south poor condition site and (D) south good condition site from January 2010. 3.3 Methods 3.3.1 Site characteristics Photographic and core samples were used to monitor short-term temporal trends in good and poor condition M. modiolus communities, in the north and south basin of Strangford Lough. The sites chosen for the surveys in the north basin were north east of Long Sheelah and for the south basin the area east of Black Rocks (Figure 3.2). For the purposes of the present study in Strangford Lough, „good‟ condition M. modiolus reefs were defined as sites with ≥ 5 individuals, and ≥ 1 clump m -2 and „poor‟ condition M. modiolus reefs were defined as sites with < 5 individuals, and < 1 clump m-2. Locating the good condition M. modiolus sites proved very difficult and time consuming and hence sampling times differed at the survey sites (see section 3.3.2. below for details). Because of the patchy nature of the M. modiolus communities, quadrats without live individuals of M. modiolus but within its historical range in the good and poor condition sites were also monitored during the surveys. 60 Sites were characterised by soft substrata with varying proportions of shell fragments. The sampling was conducted at 20-28 metres depth. During the survey period the sea surface temperature, salinity and turbidity varied between 3 - 18° C, 26 - 34 PSU (Practical Salinity Units) and 0 - 0.8 FTU (Formazin Turbidity Unit ) respectively in the northern basin (AFBI Station ID SL03) (AFBI 2011). Figure 3.2 Location of the good and poor condition sites in the north basin and south basins in Strangford Lough. The sites chosen for the surveys in the north basin were north east of Long Sheelah and for the south basin the area east of Black Rocks. NG = north good site; NP = north poor site; SG = south good site; SP = south poor site. 61 3.3.2 C C Sampling strategy D At each survey site, permanent markers were established using surface and subsurface buoys. A 30 x 30 m area around these markers was sampled, using a randomly generated table of fin kicks and compass bearings. The divers were guided from the buoys to these positions using through water communications and the Sonardyne Ultra Shortwave Baseline Location (USBL) beacon relay. Monitoring of the sites involved both photo quadrats and removal sampling using cores. 3.3.3 Monitoring undisturbed communities and densities of M. modiolus: photo quadrats and in situ counts To sample the undisturbed communities at each survey site divers took 15 replicate overhead photographs of 0.5 x 0.5 m quadrats using a Nikon D60, Nikkor 12-24 mm lens and 2 x Ikelite D125 strobes and where possible oblique photographs were also taken. The numbers of live adult M. modiolus (≥ 5 cm) in each quadrat were then counted in situ. The macrofauna present in undisturbed photoquadrats were identified from notes in the field and photo analyses in the laboratory. All photo analyses were checked by a second observer. The good condition, north basin; poor condition, north basin; and poor condition south basin; were surveyed on four occasions, (June 2008, January 2009, June 2009, January 2010), and the good condition, south basin site was surveyed on three occasions (January 2009, June 2009, January 2010). 3.3.4 Monitoring of M. modiolus epifauna, crevice and sediment infauna: core sampling To undertake more detailed sampling of the epifauna and crevice and sediment infauna, divers hammered a plastic corer of 10 cm diameter, 10-15 cm into the sediment, in the centre of the 0.5 x 0.5 m quadrat. The contents of the core were carefully transferred into a plastic bag. All samples were returned to the laboratory, washed, sieved (1 mm mesh), identified and counted. Fifteen core samples were 62 taken from all sites surveyed in January 2009 and from good and poor condition sites in the north basin in June 2009. However, due to time constraints, the number of replicates was reduced to 10 core samples in the good and poor condition south basin sites in June 2009. 3.3.5 Analyses The changes in the density of M. modiolus and the number and abundances of species in the good and poor condition reefs through time were analysed using 3way ANOVAs. The model included the main effects of condition (fixed, 2 levels = good and poor), basin (fixed, 2 levels = north basin and south basin) and time (fixed, 4 levels = June 08, January 09, June 09 and January 10 months for photographs or 2 levels = January 09 and June 09 months for cores). Boxcox plots were used to determine the appropriate transformation to stabilize variances, and transformed data were checked for both normality (using normal probability plots) and homoscedasticity. Variables that were transformed are expressed in terms of the untransformed variable Y. All univariate statistical analyses and graphics and multivariate graphics were produced using the R statistical software (www.Rproject.org). The changes in benthic community structure in the good and poor condition sites through time were analysed using a 3-way PERMANOVA calculated from a Bray-Curtis similarity matrix based on species presence/absence. These data were plotted using non-metric multidimensional scaling (nMDS) ordinations. Where significant differences were found, similarity percentage (SIMPER) analyses were used to see which taxa contributed the most to the dissimilarity. All multivariate statistical analyses were undertaken using the Primer 6.0 software with the PERMANOVA extension (Clarke & Warwick 2001; Anderson et al. 2008). 63 3.4 Results 3.4.1 Density of M. modiolus: in situ counts The mean densities of M. modiolus were significantly higher in the good condition sites than the poor condition sites through time (Table 3.1& Figure 3.3). With the exception of surveys conducted in January 2009, mean densities of M. modiolus in the good and poor condition north basin sites were higher through time than in good and poor condition sites respectively in the south basin (Table 3.1& Figure 3.3). There were no significant temporal differences in the densities of M. modiolus in good and poor sites in either basin and no significant interaction effects (Table 3.1). Table 3.1 Results of 3-way ANOVA testing the effect of condition and area on the density of M. modiolus through time from in situ counts (n = 15) in Strangford Lough. Significant p-values are shown in bold. Factors Condition Basin Time Condition x Basin Condition x Time Basin x Time Condition x Time x Basin Error df 1 1 3 1 1 3 3 42 Mean squares 215.88 22.86 0.77 6.36 1.18 0.70 0.14 0.49 f-value 435.12 46.4 1.56 12.92 2.39 1.43 0.29 p-value <0.001 <0.001 >0.05 <0.001 >0.05 >0.05 >0.05 64 20 Mean number of M. modiolus (± SE) 18 16 14 12 GN 10 PN 8 GS PS 6 4 2 0 Jun-08 0 Jan-09 6 Jun-09 12 Jan-10 18 Figure 3.3 Mean density (±SE) of M. modiolus through time (months) based on in situ counts in 0.25 x 0.25 m quadrats (n = 15). Codes are: GN = good condition, north basin; PN = poor condition, north basin; GS = good condition, south basin; PS = poor condition, south basin. 3.4.2 Photo quadrat monitoring of undisturbed communities In total 25 epifaunal species were identified from the photo quadrat analysis and field notes. Mean numbers of epifauna recorded in photo quadrats showed significant differences between good and poor sites, between the north and south basins and through time (Table 3.2 and Figure 3.4). In general, the mean numbers of epifaunal species in the good and poor sites in the north basin were higher than good and poor sites respectively in the south basin (Figure 3.4). 65 Table 3.2 Results of 3-way ANOVA testing the differences in the number of macrofauna in good and poor sites in the north and south basin through time in Strangford Lough, using photoquadrats (n = 15). Significant p-values are shown in bold. Mean number of species (±SE) Factors Condition Basin Time Condition x Basin Condition x Time Basin x Time Condition x Time x Area Error df 1 1 3 1 3 3 3 210 Mean squares 5.678 44.213 3.260 4.175 0.785 1.624 0.365 0.265 f-value 21.389 166.555 12.281 15.723 2.846 6.118 1.376 p-value <0.001 <0.001 <0.001 <0.001 > 0.05 <0.001 >0.05 6 5 4 GN 3 GS 2 PN 1 PS 0 Jun-08 1 Jan-09 Jun-09 2 3 Time (months) Jan-10 4 Figure 3.4 Mean (±SE) numbers of epifaunal species through time (months) based on 0.5 x 0. 5 m photo quadrats (n = 15). Codes are: GN = good condition, north basin; PN = poor condition, north basin; GS = good condition, south basin; PS = poor condition, south basin. There were significant differences in epifaunal species composition between communities in the good and poor sites, between the north and south basins, and through time (Table 3.3 and Figure 3.5). The nMDS plot showed that the epifauna in the good condition sites in both the north and south basins and in the poor condition site in the north remained relatively stable through time. In contrast, the epifauna in 66 the poor condition site in the south basin showed no predictable patterns through time (Figure 3.5). Table 3.3 Results of 3-way PERMANOVA testing the differences in the macrofauna community structure between good and poorBsites, in the north and south basin and B through time using 0.5 x 0.5 m photo quadrats (n=15). Significant p-values are shown in bold. Factors Condition Basin Time Condition x Basin Condition x Time Basin x Time Condition x Time x Basin Error df 1 1 3 1 3 3 3 153 Mean squares 25098 36565 11362 9474.7 7597.8 868.74 2383.5 630.14 f-value 20.565 58.026 9.310 7.764 6.226 0.712 3.7825 p-value 0.001 0.001 0.001 0.001 0.001 >0.05 0.014 67 Figure 3.5. A) nMDS ordination of the temporal changes in epifauna recorded in 0.5 x 0.5 m photo quadrats in good and poor M. modiolus reefs (n = 15) in Strangford Lough, Northern Ireland. Codes are: upright green triangles (1) = June 08, downward dark blue triangles (2) = January 09, light blue squares (3) = June 09, red diamonds (4) = January 10; GN = good condition, north basin; PN = poor condition, north basin; GS = good condition, south basin; PS = poor condition, south basin. The analysis is based on a Bray-Curtis matrix of presence/absence data. Sites from the poor condition sites show greatest spread on this nMDS plot. The encircled area in the centre of the plot contains the tightly clumped good condition sites. The cloud has been expanded and is represented in image B); Codes are: upright green triangles = north basin; downward blue triangles = south basin; (1) = June 08, (2) = January 09, (3) = June 09, (4) = January 10. The analysis is based on a Bray-Curtis matrix of presence/absence data. 68 A B 69 SIMPER analysis showed that 6 species contributed most to the differences observed between good and poor sites in the north and south basins (Table 3.3). The north and south basin good condition sites were characterised by a high frequency of occurrence of Antedon bifida, Ophiocomina nigra, and Thyone spp. relative to the poor condition sites (Table 3.4). There was higher frequency of occurrence of all macrofauna in both the good and poor condition sites in the north basin than either site in the south basin (table 3.4). Table 3.4 SIMPER analyses showing the macro-epifauna species contributing to 70% of the dissimilarity within and between the good and poor condition sites in the north and south basins of Strangford Lough, using 0.5 x 0.5 m photo quadrats (n = 15). North basin: Good vs Poor condition Average similarity = 55.48 Modiolus modiolus Ophiocomina nigra Antedon bifida Ascidiella aspersa Crisia spp. Thyone spp. South basin: Good vs. Poor Average dissimilarity = 72.72 Modiolus modiolus Antedon bifida Ophiocomina nigra Ascidiella aspersa Good Poor condition condition Average Average frequency of frequency occurrence of occurrence 0.97 0.53 0.69 0.63 0.69 0.82 0.29 0.07 0.25 0.07 0.20 0.10 Cumulative (%) Good Poor condition condition Average Average frequency of frequency occurrence of occurrence 0.85 0.38 0.39 0.32 0.39 0.32 0.24 0.05 Cumulative (%) 18.47 34.33 49.70 63.47 63.47 70.19 32.67 47.62 61.10 66.80 70 3.4.3 M. modiolus epifauna, crevice and sediment infauna: core sampling The nMDS plot of M. modiolus communities sampled by coring suggested little temporal change in communities in good sites in both the north and south basins and in those from poor sites in the north basin (Figure 3.6). In contrast, the fauna in the cores in the poor M. modiolus beds in the south showed a distinctive shift in MDS space after 6 months (Figure 3.6) which is reflected in significant effects of time and time-related interactions (Table 3.5). Figure 3.6 nMDS ordination of the temporal trends in good and poor sites in the north (n = 15, n = 15), and south basin (n = 15, n = 10) in Strangford Lough, Northern Ireland. Codes are: upright triangles GN = good condition, north basin; downward triangles PN = poor condition, north basin; circles GS = good condition, south basin; diamonds PS = poor condition, south basin, January 09 = blue, June 09 = green. The analysis is based on a Bray-Curtis matrix of square root transformed count data. 71 Table 3.5 Results of PERMANOVA testing the differences in the fauna community structure between good and poor sites, in the north (n = 15, n = 15) and south (n = 15, n = 10) basin and through time using cores. Significant p-values are shown in bold. Factors Condition Basin Time Basin x Area Condition x Time Basin x Time Condition x Time x Basin Error df 1 1 1 1 1 1 1 101 Mean squares 43062 45931 15333 16004 18885 14612 11136 1740.6 f-value 2.280 3.144 8.809 1.437 10.85 8.395 6.398 p-value >0.05 >0.05 0.001 >0.05 0.001 0.001 0.001 SIMPER analyses showed that 21 species contributed to the differences between good and poor sites in the north and south basin and most of these species were infaunal (Table 3.6). The north good condition site was characterised by high abundances of Pisidia longicornis, Modiolus modiolus, Abra alba and Ophiothrix fragilis relative to the poor condition site (Table 3.7). In contrast, the good condition south site also had a higher abundance of M. modiolus but lower abundance of P. longicornis, Thoralus cranchii and A. alba relative to the poor condition site (Table 3.6). 72 Table 3.6 SIMPER analyses showing the species contributing to 70% of the dissimilarity within and between good and poor M. modiolus communities sampled by coring in the north (n =15, n = 15) and south (n =15, n = 10) basins through time, Strangford Lough. North basin Good vs. Poor Average similarity = 68.53 Pisidia longicornis Modiolus modiolus Abra alba Ophiothrix fragilis Nucula nucleus/sulcata Antedon bifida Lepidonotus squamatus Ophiocomina nigra Venerupis senegalensis Pomatoceros spp. Tapes (=Venerupis) rhomboides Pherusa plumose Ascidiella aspersa Good Average abundance 4.94 1.51 1.75 1.57 1.95 0.15 0.83 0.57 0.56 0.44 0.40 0.42 0.38 Good Average similarity 2.11 0.08 0.68 0 2.22 0.84 0.34 0.38 0.42 0.28 0.22 0.22 0 Cumulative (%) South Good vs. Poor Average similarity = 90.16 Good Poor condition condition Average Average frequency of similarity occurrence 1.70 0.03 0 2.23 0.74 1.05 1.00 0.23 0.83 0.07 0 0.56 0 0.77 0.51 0.27 0.51 0.21 0.49 0.18 0.33 0.25 0 0.37 0.15 0.14 Cumulative (%) Modiolus modiolus Thoralus cranchii Pisidia longicornis Abra alba Crisia denticulate Nucula nucleus/sulcata Boreotrophon truncates Sthenelais boa Pherusa plumose Nephtys incise Harmothoe spp. Pomatoceros spp. Tapes (=Venerupis) rhomboides 17.43 25.07 32.40 39.69 46.60 51.05 55.07 58.43 61.58 64.12 66.53 68.78 70.86 11.34 22.20 30.69 36.19 41.63 50.34 54.04 57.72 61.27 64.50 67.67 69.37 71.56 73 In total, 79 species were recorded in the core samples. Species numbers and abundances were highly variable and showed no clear patterns. Overall there were significant differences between the numbers and abundances of species in coresamples from good and poor sites, from the north and south basins, and the different sampling times (Figures 3.7 & 3.8 and Tables 3.7 & 3.8). The mean numbers of species in both the good and poor condition sites in the north basin were lower in June 2009 than in January 2009, (fig. 3.7). In contrast, the mean numbers of species in both the good and poor condition sites in the south basin were lower in January 2009 than in June 2009 (fig. 3.7). These differences could partly be explained by the high spatial seasonal variability in infaunal species. Mean number of species (± SE) 14 12 10 8 GN 6 PN 4 GS 2 PS 0 6 Jan-09 Jun-09 Time (months) Figure 3.7 Mean number (±SE) of fauna in good and poor condition sites in north (n =15, n =15) and south basins (n =15, n =10) in cores through time (months). Codes are: GN = good condition, north basin; PN = poor condition, north basin; GS = good condition, south basin; PS = poor condition, south basin. 74 Table 3.7 Results of 3-way ANOVA testing the differences in the number of fauna in cores in good and poor M. modiolus reefs in the north (n =15, n =15) and south basin (n =15, n =10) through time in Strangford Lough. Significant p-values are shown in bold. Factors Condition Basin Time Condition x Basin Condition x Time Basin x Time Condition x Time x Basin Error df 1 1 1 1 1 1 1 101 Mean squares 4.1532 1.9298 0.2086 0.8068 0.1321 7.1427 0.0066 0.2320 f-value 17.8990 8.3167 0.8988 3.4771 0.5693 30.7826 0.0284 p-value <0.001 <0.001 >0.05 >0.05 >0.05 <0.001 >0.05 There were significantly lower mean abundances of species in good condition sites in the north basin and both the good and poor condition sites in the south basin in January 2009 than in June 2009, (Figure 3.8 and Table 3.8). In contrast, the mean abundances of species were significantly higher in the poor condition site in the north Mean abundances of species (± SE) in January 2009 than in June 2009 (Figure 3.8 and Table 3.8). 70 60 50 40 GN 30 PN 20 GS 10 PS 0 6 Time (months) Figure 3.8 Mean abundances (± SE) of fauna in cores in good and poor condition sites in the north (n = 15, n = 15) and south basins (n =15, n = 10) through time (months). Codes are: GN = good condition, north basin; PN = poor condition, north basin; GS = good condition, south basin; PS = poor condition, south basin. 75 Table 3.8 Results of 3-way ANOVA testing the differences in the abundances of fauna in cores in good and poor M. modiolus reefs in the north (n =15, n = 15), and south (n =15, n = 10) basin through time in Strangford Lough. Significant p-values are shown in bold. Factors Condition df 1 Mean squares 4.1532 f-value 17.8990 p-value >0.001 Basin Time 1 1 1.9298 0.2086 8.3167 0.8988 0.004 >0.05 Condition x Basin Condition x Time 1 1 0.8068 0.1321 3.4771 0.5693 > 0.05 >0.05 Basin x Time Condition x Time x Basin Error 1 1 101 7.1427 0.0066 0.2320 30.7826 0.0284 <0.001 >0.05 3.5 Discussion The present study showed sites with a higher abundance of Modiolus modiolus had a higher abundance of associated macrofauna. This agrees with other studies of M. modiolus communities (biotopes SS.SBR.SMus.Mod.Car, SS.SBR.SMus.ModHAs and SS.SBR.SMus.ModT) in Scotland and Wales (Holt et al. 1998, Rees et al. 2008, Sanderson et al. 2008). Species associated with the M. modiolus communities included, epifaunal species Antedon bifida; Ophiocomina nigra; Obelia spp.; Thyone spp. and T. drummondii; Crisia spp.; and Alcyonium digitatum and infaunal species Abra alba and Mysella bidentata (Holt et al. 1998; Rees et al. 2008; Sanderson et al. 2008). The present study revealed substantial differences between both the taxa recorded and temporal differences identified using photoquadrat and core samples. Photoquadrats confirmed that good condition sites had a higher abundance of M. modiolus and associated macrofauna than poor sites. In contrast, there were no predictable differences between the epifauna and crevice and sediment infauna taxon between good and poor condition sites in the cores. These results suggest that 76 the structure of M. modiolus clumps can have very different influences on epifauna and infauna. Like other studies on M. modiolus communities (Rees et al. 2008; Sanderson et al. 2008) the present study found high spatial and temporal variability in the fauna recorded from photoquadrats and cores. In general, good condition sites remained much more stable through time relative to poor condition sites. The poor condition site in the south basin, which had the lowest density of M. modiolus and mean number of epifaunal species, showed the greatest variability in community structure through time. The variation in fauna probably reflects the high variation in the differences between samples with and without M. modiolus clumps within the plots. Epifaunal diversity was significantly higher in the north than in the south basin. This difference reflects differences between M. modiolus biotopes, SS.SBR.SMus.ModCvar in the north is characteristically more species rich than and SS.SBR.SMus.ModHAs and SS.SBR.SMus.ModT in the south. These differences reflect environmental differences between the two basins (Roberts et al. 2004). The number of macrofaunal taxa recorded in the present study using photo quadrats was similar to that reported by Magorrian and Service (1998). The major difference was the complete absence of Chlamys varia and Aequipecten opercularis and the lower abundances of Alcyonium digitatum in our photographs compared with those taken in previous surveys by Erwin et al. (1986) and Magorrian & Service (1998). These differences indicate that the impacted communities, even at the better sites, have not yet fully recovered. During the present study there were no detectable changes in the densities of M. modiolus or its associated fauna in either good or poor condition sites which suggests that temporal monitoring during this project has not been carried out long enough for changes to be picked up. Studies on Modiolus communities in New Zealand and Canada suggest they may take decades to recovery from anthropogenic impacts (Cranfield et al. 2004; Kenchington et al. 2007). To better understand natural recovery rates and species succession in M. modiolus communities future monitoring should be carried out on targeted sites and no more frequently than at annual intervals, to minimise seasonal variability. 77 4.0 Potential for natural recovery of M. modiolus communities (Undertaking 6) 4.1 Summary In 2003, the Modiolus modiolus communities in Strangford Lough were surveyed in various historical sites in the north and south basins to sample the M. modiolus with Chlamys varia, biotope (code SS.SBR.SMus.ModCvar) and the M. modiolus with hydroids biotope (SS.SBR.SMus.ModHAs) in the north and south basins respectively. Samples were compared using dive and video transects and by detailed analysis of samples removed from 0.25m2 quadrats. In this part of the current project surveys were repeated in 2010 using the same methodology and at the same sites, as far as was possible, to determine the long term potential for natural recovery of M. modiolus communities. In the north basin sites there have been further declines in the density and frequency of occurrence of M. modiolus, relative to 2003 surveys. In the south basin there have been further declines in the density of M. modiolus, but increases in the frequency of occurrence of M. modiolus suggesting greater fragmentation of the biotope, relative to 2003 surveys. There was a decrease in the mean number of species and Shannon‟s and Pielou‟s diversity indices between 2003 and 2010 in the north basin. There was an increase in the mean number of species but no clear differences in Shannon‟s and Pielou‟s diversity indices between 2003 and 2010 in the south basin. This part of the study suggests that the biotope in the north basin continues to decline in condition, whereas the biotope in the south basin appears to show increased fragmentation although some good condition sites remain. 78 4.2 Introduction In the 1950s, M. modiolus was reported to be „commonly dredged in many localities in the Lough‟ (Williams 1954). The M. modiolus communities in Strangford Lough were first described in the early 1970s and 1980s (Roberts 1975; Erwin 1986). Surveys and removal sampling during this time identified two main communities, Modiolus modiolus with Chlamys varia, sponges, hydroids and bryozoans biotope (code SS.SBR.SMus.ModCvar) in the north basin with 90 associated species (Brown and Seed, 1977; Erwin et al. 1990) and M. modiolus with hydroids and large solitary ascidians in the south basin (SS.SBR.SMus.ModHAs), with 84 associated species (Roberts 1975; Erwin et al. 1990). The focus of these surveys was to document the dominant species on the M. modiolus communities in Strangford Lough (Roberts 1975) and to map their distribution (Erwin et al. 1990). Side scan sonar surveys in the 1990s suggested that M. modiolus communities in Strangford Lough had been damaged by mobile fishing gear (Magorrian et al. 1995; Service and Magorrian 1997; Magorrian and Service 1998). However, surveys and removal sampling of M. modiolus communities in 2003 recorded higher numbers of species in the SS.SBR.SMus.ModCvar biotope than previous surveys and similar numbers on the SS.SBR.SMus.ModHAs biotope (Roberts 1975; Roberts et al. 2004). These differences are likely to be explained by a number of factors including improved taxonomic skills, and increased sampling effort. The use of mobile fishing gear in Strangford Lough was banned by DARD in 2003. Because the decline in the distribution and density of M. modiolus has continued in some areas (Section 2) and communities showed no clear short-term trends (Section 3) the current frequency of occurrence and density of M. modiolus and species composition of M. modiolus biotopes was compared with those recorded in 2003 during the Strangford Lough Environmental Change Investigation (SLECI). During the current project, surveys were repeated at the same sites sampled by SLECI in 2003. Data were also compared with surveys undertaken in 2007 at other sites by NIEA and the Ulster Museum. It was considered that this gave the greatest opportunity to pick up potential recovery of M. modiolus communities after the trawling ban was introduced in December 2003. 79 The aim of this part of the project was to assess the potential for natural recovery of impacted M. modiolus communities in Strangford Lough by comparing data from MRRG surveys with those reported in SLECI (Roberts et al. 2004). Specific objective were to: Investigate changes in the frequency of occurrence of M. modiolus between 2003 and 2010, using video surveys. Investigate changes in community structure in M. modiolus biotopes, between 2003 and 2007, using video surveys. Investigate changes in the density of mussels and community structure in M. modiolus biotopes between 2003 and 2010, using removal quadrat sampling. 4.3 Methods 4.3.1 Transect surveys – Changes in frequency of occurrence of M. modiolus between 2003 and 2010 In 2010, 100 m transect surveys were undertaken by divers to monitor the temporal changes in the frequency of occurrence of M. modiolus. The surveys were conducted at 10 sites previously surveyed in 2003 (Roberts et al. 2004; Figure 4.1): Green Island, Jane‟s Rock, south Hadd Rock 1, south Hadd Rock 2, south Hadd rock 3, north west Long Sheelah, Scott‟s Hole, Black Rocks, west Round Island Pinnacle and Slave Rock (Figure 4.1). At each site, a lead line transect rope was deployed using concrete blocks and shot lines. Two divers descended the shotline; one diver took notes on presence and absence of live M. modiolus at 5 m intervals along the transect, while the other diver took photographs. All photos were viewed by a minimum of two observers to ensure consistency of species identification and estimates of abundances. Due to differences in methodology these surveys of epifauna were only used to make in situ observations. Three replicates were taken at each site in 2010; in 2003 surveys were not replicated. This was essentially the same sampling strategy used in 2003 except that the current surveys involved greater replication. 80 4.3.2 Transect surveys – Changes in M. modiolus abundance and communities between 2003 and 2007 In 2007, 100 m transect surveys were undertaken by NIEA and the Ulster Museum to monitor the abundances of M. modiolus and the epifauna at the following 7 sites: Green Island (n = 1 in 2003, n = 1 in 2007), Jane‟s Rock (n = 1 in 2003, n = 1 in 2007), south Hadd Rock (n = 1 in 2003, n = 2 in 2007), north west Long Sheelah (n = 4 in 2003, n = 2 in 2007), north west Long Sheelah (n = 1 in 2003, n = 1 in 2007), Black Rocks (n = 5 in 2003 n = 3 in 2007) and west Round Island Pinnacle (n = 3 in 2003, n = 1 in 2007) (fig. 4.1). For each survey, 1 diver recorded the presence of epifaunal species while the other diver videoed the dive. These videos were used to determine the abundance of species using the SACFOR scale (JNCC 1990). The number of surveys was n = 18 in 2003 and n = 11 in 2007, see specific details above. Figure 4.1 Location of the transect surveys in 2010 at Green Island, Jane‟s Rock, south Hadd Rock, north west Long Sheelah, Scott‟s Hole, Black Rocks, west Round 81 Island Pinnacle, and Slave Rock Strangford Lough. Sites were originally surveyed in SLECI 2003 and in 2007 by NIEA and the Ulster Museum. 4.3.3 Removal quadrats – Changes in M. modiolus densities and communities between 2003 and 2010 To investigate changes in the densities of M. modiolus and community structure in M. modiolus biotopes, 2 divers collected all mussels and underlying sediment samples from 5 randomly thrown 0.5 x 0.5 m quadrats in M. modiolus communities, at 4 sites: south Hadd Rock, north west Long Sheelah, Black Rocks and west Round Island Pinnacle (Figure 4.2). These sites were chosen based on sites sampled in 2003 (Figure 4.2, Roberts et al. 2004) and to include both SS.SBR.SMus.ModCvar and SS.SBR.SMus.ModT) biotopes. All samples were collected by shovelling the mussels and sediment (~100 mm depth) into 1 mm mesh bags. These bags were sent to the surface using lift bags and transported to the laboratory in seawater. At the laboratory, the samples were fixed in 4% formalin for 48h, after which they were washed through a 1mm sieve. Fauna were sorted into major phyla and preserved in 70% ethanol. All taxa were identified to species level where possible and counted. All these samples have been retained as a voucher collection which will be retained by Queen‟s University until they can be accessioned to the Ulster Museum collections. 82 Figure 4.2 Location of the removal quadrats at south Hadd Rock, north east Long Sheelah, Black Rocks and west of Round Island Pinnacle in Strangford Lough surveyed during the present study. 4.3.4 Analyses 4.3.4.1 Transect surveys – Changes in frequency of occurrence of M. modiolus between 2003 and 2010 The presence live M. modiolus at 5 m intervals were converted to percentage occurrence of mussels along the 100 m transects. Differences in frequency of occurrence of M. modiolus between 2003 and 2010 were analysed using a 2-way ANOVA. The model included the main effects of time (fixed, 2 levels = 2003 and 2010), and site (fixed, 10 levels = Green Island, Jane‟s Rock, south Hadd Rock 1, south Hadd Rock 2, south Hadd rock 3, north west Long Sheelah, Scott‟s Hole, Black Rocks, west Round Island Pinnacle and Slave Rock). 83 4.3.4.2 Transect surveys – Changes in community structure in M. modiolus biotopes between 2003 and 2007 The SACFOR estimates of M. modiolus and epifaunal abundances along the 100 m transect were converted to numbers using the mean from the JNCC SACFOR tables. These data were log10(x+1) transformed to minimise the errors introduced by estimating abundances. Changes in the mean abundances and number of epifaunal taxa were analysed using 2-way ANOVAs. The model included the main effects of time (fixed, 2 levels = 2003 and 2007) and site (fixed, 2 levels = north basin SS.SBR.SMus.ModCvar and south basin SS.SBR.SMus.ModHAs). The changes in the epifaunal communities through time were analysed using PERMANOVA (model details above) calculated from a Bray-Curtis similarity matrix. All rare species found in < 1% of the samples were excluded from the analyses, and all species difficult to identify were pooled to the level of genera. These data were plotted using non-metric multidimensional scaling (nMDS) ordinations. Where significant differences were found similarity percentage (SIMPER) analyses were used to see which taxa contributed the most to the dissimilarity. 4.3.4.3 Removal quadrats – Changes in M. modiolus densities and communities between 2003 and 2010 Differences in the density of M. modiolus, and numbers of epifaunal, crevice and sediment infaunal taxa at different sampling times were analysed using 2-way ANOVAs. The model included the main effects of time (fixed, 2 levels = 2003 and 2010), and sites (fixed, 4 levels = south Hadd Rock, north west Long Sheelah, Black Rocks and west Round Island Pinnacle). Temporal changes in communities were analysed using PERMANOVA (model details above) calculated from a Bray-Curtis similarity matrix based on species presence/absence data. All rare species found in < 1% of the samples were excluded from the analyses and all species difficult to identify in the field were pooled to the level of genera. These data were plotted using non-metric multidimensional scaling (nMDS) ordinations. Where significant differences were found similarity percentage (SIMPER) analyses were used to see which taxa contributed the most to the dissimilarity. 84 For all univariate analyses, boxcox plots were used to determine the appropriate transformation to stabilize variances, and transformed data were checked for both normality (using normal probability plots) and homoscedasticity (homogeneous variance). Variables that were transformed are expressed in terms of the untransformed variable Y. Univariate statistical analyses and graphics were produced using the R statistical software (www.R-project.org). Multivariate statistical analyses and graphics were undertaken using the Primer 6.0 software with the PERMANOVA extension (Clarke & Warwick 2001; Anderson et al. 2008). 4.4 Results 4.4.1 Transect surveys – Changes in frequency of occurrence of M. modiolus between 2003 and 2010 Of the 10 sites surveyed, there were significant declines in the frequency of occurrence of M. modiolus between 2003 and 2010 at 6 sites: south Hadd Rock 1, south Hadd Rock 2, south Hadd Rock 3, north west Long Sheelah, Scott‟s Hole, and Green Island between 2003 and 2010 (Figure 4.3 and Table 4.1). At 2 sites, Black Rocks and west Round Island Pinnacle, there was an increase in the frequency of occurrence of M. modiolus between 2003 and 2010 (Figure 4.3 and Table 4.1). At the 2 remaining sites, Jane‟s Rock and Slave Rock no mussels were recorded on either date (Figure 4.3 and Table 4.1). Table 4.1 Results of 2-way ANOVA testing the changes in the frequency of occurrence of M. modiolus between 2003 (n = 1) and 2010 (n = 3) at 10 sites in Strangford Lough. Significant p-values are shown in bold. Factors Time Site Time x Site Error df 1 8 8 18 Mean Square 59.895 90.323 12.641 3.197 f-value 18.734 28.251 3.954 p-value < 0.001 < 0.001 < 0.001 85 % of M. modiolus along 100 m transects (± SE) d) NW Long Sheelah e) Scott’s Hole f) Jane’s Rock f) Scott’s Hole g) Slave Rock h) Black Rocks f) Scott’s Hole f) Scott’s Hole i) W Round Island Pinnacle j) Green Island f) Scott’s Hole Time (years) Figure 4.3 Changes in the mean (± SE) frequency of occurrence of M. modiolus at 10 sites in Strangford Lough between 2003 (n =1) and 2010 (n = 3). 86 4.4.2 Transect surveys – Changes in community structure in M. modiolus biotopes between 2003 and 2007 In total 120 species were identified in the transect surveys in 2007. There were no significant differences in the mean numbers of species of epifauna between north and south basin or through time (Figure 4.4 and Table 4.2). However, epifaunal communities showed significant differences through time and between basins (Figure 4.5 and Table 4.3). The nMDS plot shows the separation between the north and south basins, in 2003 and 2007. Figure 4.4 Changes in the (± SE) mean number of epifauna along 100 m transects between 2003 and 2007 in the north and south basin in Strangford Lough (n = 18, 2003, n = 11, 2007). Table 4.2 Results of 2-way ANOVA testing the differences in the mean number of epifauna along 100 m transects between 2003 and 2007 in the north and south basin sites in Strangford Lough (n = 18, 2003, n = 11, 2007). Factors Year Basin Year x Basin Error 1 6 6 16 Mean Square 81.000 917.200 141.161 227.985 f-value 0.6705 0.356 0.620 p-value > 0.05 > 0.05 > 0.05 87 Figure 4.5 nMDS ordination of the changes in M. modiolus epifauna between 2003 and 2007 in the north and south basin in Strangford Lough, Northern Ireland. The analysis is based on a Bray-Curtis matrix of log (x+1) transformed abundance data (n = 18, 2003, n = 11, 2007). Upright green triangles = 2003 and downward blue triangles = 2007. N = north basin sites and S = south basin sites. Table 4.3 Results of 2-way PERMANOVA testing the differences in the epifauna community structure between 2003 and 2007 using 100 m transects in the north and south basins in Strangford Lough, (n =18, 2003, n = 11, 2007). Significant p values are shown in bold. Factors Year Basin Year x Basin Error df 1 1 1 26 Mean square 4746.2 4845.5 2543.9 1378.8 f-ratio 3.444 3.5143 1.845 p-value 0.001 0.001 0.019 SIMPER analyses showed that 42 species contributed to 70 % of the dissimilarity between 2003 and 2007 (Table 4.4). In the north basin 35 species contributed to 88 70% of the dissimilarity between 2003 and 2007. There were significant declines in the abundances of Chlamys varia, Aequipecten opercularis, Ascidiella aspersa, Obelia dichotoma, Halecium halecinum, Kirchenpaueria pinnata between 2003 and 2007. In the south basin 48 species contributed to 70 % of the dissimilarity between 2003 and 2007. There were significant declines in the abundances of Ascidiella aspersa and increases in the abundances of Ophiura albida and Halecium halecinum between 2003 and 2007. 89 Table 4.4 Epifauna community. Results of SIMPER analyses showing the species contributing to 70% of the dissimilarity between 2003 and 2007 in the north and south basins in 100 m transects (n =18, 2003 and n = 11, 2010). North basin 2003 vs. 2007 Average dissimilarity = 55.86 Chlamys varia Ascidiella aspersa Pomatoschistus pictus Necora puber Ophiothrix fragilis Aequipecten opercularis Thyone roscovita Echinus esculentus Obelia dichotoma Balanus spp. Alcyonidium diaphanum Scrupocellaria scruposa Thyone fusus Serpula vermicularis Ostrea edulis Buccinum undatum Carcinus maenas Inachus dorsettensis Ophiocomina nigra Macropodia rostrata Halecium halecinum Inachus phalangium Sertularella polyzonias Modiolus modiolus Kirchenpaueria pinnata Liocarcinus correcatus Cellepora pumicosa Pandalus montagui Alcyonium digitatum Cliona celata Inachus phalangium Calliostoma zizyphinum Amphilectus fucorum Clavelina lepadiformis Mycale spp.spp. Alcyonidium diaphanum 2003 Average abundance 2.39 2.04 1.97 0.61 2.99 2.02 2.07 1.19 1.29 1.24 1.57 1.40 1.62 1.47 1.50 1.81 0.48 1.43 1.95 1.50 1.88 1.09 1.19 3.29 1.26 1.09 1.19 0.85 1.09 0.76 0.70 0.67 0.76 0.67 0.58 0.83 2007 Average abundance 0.18 0.18 0.40 2.41 2.61 0.35 1.29 2.42 0.86 0.98 0 0.31 0.28 0 0.97 1.40 1.40 1.80 2.48 1.01 1.59 0.79 0.67 2.73 0.66 0.66 0.18 0.70 0.79 0.56 0.50 0.56 0.11 0.56 0.51 0.31 Cumulative (%) 2.68 5.18 7.53 9.80 12.08 14.23 18.52 20.42 22.32 24.10 25.82 27.52 29.21 30.87 32.52 34.16 35.78 37.36 38.92 40.47 42.02 43.56 45.00 46.52 47.82 49.15 50.46 51.77 53.07 65.40 62.25 65.40 64.37 67.44 68.41 69.32 90 South basin 2003 vs. 2007 Average dissimilarity = 59.87 2003 Average abundance 2007 Average abundance Cumulative (%) Ascidiella aspersa Ophiura albida Pomatoceros spp.spp. Cerianthus lloydii Sagartia troglodytes Thyone roscovita Pomatoschistus pictus Halecium halecinum Ophiothrix fragilis Echinus esculentus Balanus spp. Mycale spp. Urticina eques Pecten maximus Cancer pagurus Necora puber Doto spp. Antedon bifida Leptasterias muelleri Carcinus maenas Pagurus bernhardus Pomatoschistus minutus Nemertesia antennina Ophiocomina nigra Alcyonium digitatum Amphilectus fucorum Sertularella polyzonias Suberites carnosus Pandalus montagui Buccinum undatum Inachus dorsettensis Scrupocellaria scruposa Calliostoma zizyphinum Macropodia rostrata Obelia dichotoma Crossaster papposus Chlamys varia Gobius niger Henricia oculata Cliona celata Nephrops norvegicus Pholis gunnellus Alcyonidium diaphanum Callionymus lyra Clavelina lepadiformis Rhizocaulus verticillatus Kirchenpaueria pinnata Cliona celata 2.63 0 0.27 0.42 0 2.38 1.61 1.23 1.42 2.27 2.17 0.61 0.80 1.57 0.54 1.23 0 2.60 1.15 0.80 0.54 0 1.42 0.88 0.96 1.07 0.80 0.88 1.07 1.07 1.42 0.54 0.96 1.34 0.80 1.76 1.07 0 1.49 0 0 0.85 0 0.58 0.54 0.35 0.27 0.54 0 2.61 1.92 2.30 1.61 1.55 0.86 2.46 1.23 1.23 2.09 1.23 1.47 0.85 1.38 1.54 1.23 2.57 0.54 1.07 1.07 1.01 1.61 0.69 1.07 0.31 1.07 0.85 0.54 0.54 1.78 0.69 0.54 1.61 0.54 1.76 0 0.94 1.61 0.85 0.85 0.63 0.85 0.54 0.54 0.69 0.69 0.54 3.30 6.57 8.87 11.12 13.19 15.24 17.26 19.06 20.77 22.44 24.10 25.10 27.22 28.73 30.23 31.66 33.09 34.49 35.89 37.25 38.59 39.93 41.27 42.59 43.90 45.21 46.51 47.80 49.09 50.37 51.64 52.86 54.08 55.30 56.46 57.61 58.77 59.71 61.05 62.18 63.31 64.37 64.37 65.40 66.42 68.41 69.38 70.34 91 4.3.3 Removal quadrats – Changes in density of M. modiolus between 2003 and 2010 There was a significant decline in the density of M. modiolus at north east Long Sheelah, south Hadd Rock and west of Round Island Pinnacle between 2003 and 2010 (Table 4.5 and Figure 4.6). In 2003, the average density of M. modiolus was 360, 288, and 318 mussels m-2, at north east Long Sheelah, south Hadd Rock and west Round Island Pinnacle, respectively. In 2010, the average density of M. modiolus had declined to 16, 136, and 37 mussels m -2, at north east Long Sheelah, south Hadd Rock and west of Round Island Pinnacle. In contrast, at the Black Rocks site there were no detectable changes in the density of M. modiolus, 16 mussels m-2, in 2003 and 2010 (Table 4.5 and Figure 4.6). Table 4.5 Results of 2-way ANOVA testing the differences in the density of M. modiolus in 0.25 x 0.25 quadrats between 2003 and 2010 at 4 sites in Strangford Lough (north east Long Sheelah n = 4 in 2003, n = 5 in 2010, south Hadd Rock n = 4 in 2003, n = 5 in 2010, Black Rocks n = 5 in 2003, n =5 in 2010 and west Round Island Pinnacle n =7 in 2003, n = 5 in 2010). Significant p-values are shown in bold. Factors Year Site Year x Site Error df 1 3 3 32 Mean Square 118.984 25.395 79.091 6.513 f-value 18.268 3.899 12.143 p-value <0.001 0.018 <0.001 92 Mean density of M. modiolus in 0.5 x 0.5 m quadrats (± SE) 2010 Time (years) Figure 4.6 Changes in the (± SE) mean number of M. modiolus in 0.25 x 0.25 m quadrats between 2003 and 2010 at four sites in Strangford Lough (north east Long Sheelah n = 4 in 2003, n = 5 in 2010, south Hadd Rock n = 4 in 2003, n = 5 in 2010, Black Rocks n = 5 in 2003, n =5 in 2010 and west Round Island Pinnacle n =7 in 2003, n = 5 in 2010). 93 4.4.3 Epifauna, and crevice and sediment infauna in quadrats In total 177 epifaunal and crevice and sediment infaunal species were identified using the 0.5 x 0.5 m removal quadrats (Appendix 4). The temporal differences in the mean number of species varied between the sites (Table 4.6 and Figure 4.7). At the site south of Hadd Rock the total number of species remained relatively constant between 2003 and 2010 whereas at the site north east of Long Sheelah the total number of species showed significant declines between 2003 and 2010 (Table 4.6 and Figure 4.7). In contrast, at the sites east of Black Rocks and west of Round Island Pinnacle there was a significant increase in the total number of species between 2003 and 2010 (Table 4.6 and Figure 4.7). Shannon diversity and Pielou evenness indices for the removal quadrat sites declined between 2003 and 2010 in the north basin but not the south basin (Table 4.7).. Table 4.6 Results of 2-way ANOVA testing the differences in the number of epifauna, crevice and sediment infauna in 0.5 x 0.5 quadrats between 2003 and 2010 at four sites in Strangford Lough.(north east Long Sheelah n = 4 in 2003, n = 5 in 2010, south Hadd Rock n = 4 in 2003, n = 5 in 2010, Black Rocks n = 5 in 2003, n = 5 in 2010 and west Round Island Pinnacle n =7 in 2003, n = 5 in 2010). Significant p-values are shown in bold. Factors Time Site Time x Site Error df 1 3 3 32 Mean squares 1.205 1.049 18.040 11.150 f-value 3.457 3.011 17.259 p-value >0.05 0.045 <0.001 94 Figure 4.7 Changes in the (± SE) mean number of epifauna crevice and sediment infauna in 0.25 x 0.25 m quadrats between 2003 and 2010 at four sites in Strangford Lough (north east Long Sheelah n = 4 in 2003, n = 5 in 2010, south Hadd Rock n = 4 in 2003, n = 5 in 2010, Black Rocks n = 5 in 2003, n =5 in 2010 and west Round Island Pinnacle n =7 in 2003, n = 5 in 2010). Table 4.7 The average Shannon diversity Pielou evenness indices in removal quadrats at 4 sites in Strangford Lough (north basin: north east Long Sheelah n = 4 in 2003, n = 5 in 2010, south Hadd Rock; south basin: n = 4 in 2003, n = 5 in 2010, Black Rocks n = 5 in 2003, n =5 in 2010 and west Round Island Pinnacle n =7 in 2003, n = 5 in 2010). Indices values were calculated using (log2) on counted species only. Indices Site North basin South Hadd Rock North east Long Sheelah South basin East Black Rock West Round Island Pinnacle Shannon diversity 2003 2010 Pielou evenness 2003 2010 2.677 2.759 1.219 1.452 0.759 0.749 0.566 0.484 2.568 2.571 2.873 3.012 0.767 0.759 0.741 0.843 95 M. modiolus communities identified in the removal quadrats in 2003 and 2010 showed significant temporal and spatial differences (Table 4.8). In 2003, the M. modiolus communities in the north basin (SS.SBR.SMus.ModCvar), (south Hadd Rock and Long Sheelah) in the south basin (SS.SBR.SMus.Mod.HAs) (east of Black Rocks and west Round Island Pinnacle) showed distinct separation in MDS space (Figure 4.8). In contrast, in 2010 the distinction between the M. modiolus communities in the north and south basins was not as clear (Figure 4.8). In 2010, the sites with the greatest separation in MDS space were north east Long Sheelah and west Round Island Pinnacle (Figure 4.8). Table 4.8 Results of PERMANOVA testing the differences in the sediment and crevice infauna community structure between 2003 and 2010 using removal quadrats at 4 sites in Strangford Lough (north east Long Sheelah n = 4 in 2003, n = 5 in 2010, south Hadd Rock n = 4 in 2003, n = 5 in 2010, Black Rocks n = 5 in 2003, n =5 in 2010 and west Round Island Pinnacle n =7 in 2003, n = 5 in 2010). Significant p-values are shown in bold. Factors Time Site Time x Site Error Df 1 3 3 32 Mean squares 25099 4514 3741 914.32 f-value 27.451 4.937 4.0916 p-value <0.001 0.001 0.001 96 Figure 4.8 nMDS ordination of the changes in M. modiolus sediment and crevice infauna in 0.5 x 0.5 m quadrats between 2003 and 2010 at four sites in Strangford Lough, Northern Ireland. Upright green triangles = 2003 and downward blue triangles = 2010. Codes are: HR = south Hadd Rock; LS = north east Long Sheelah; BR = Black Rocks; RIP = west Round Island Pinnacle. The analysis is based on a BrayCurtis matrix of presence/absence data. SIMPER analysis showed that 40 crevice and sediment infaunal taxa contributed most to the differences between 2003 and 2010 in the four sites (Table 4.9). At all sites, there were declines in the frequency of occurrence of crevice infauna and increases in the frequency of occurrence of the sediment infauna between 2003 and 2010 (Table 4.10). 97 Table 4.9 Epifauna and crevice and sediment infauna community. Results of SIMPER analyses showing the species contributing to 50% of the dissimilarity between 2003 and 2010 in 0.25 x 0.25 m removal quadrats from 4 sites in Strangford Lough (north east Long Sheelah n = 4 in 2003, n = 5 in 2010, south Hadd Rock n = 4 in 2003, n = 5 in 2010, Black Rocks n = 5 in 2003, n =5 in 2010 and west Round Island Pinnacle n =7 in 2003, n = 5 in 2010). South Hadd Rock 2003 vs. 2010 Average dissimilarity = 65.50 Buccinum undatum Chlamys varia Mytilus edulis Nephtys incisa Notomastus latericeus Pholoe synophthalmica Pomatoceros lamarckii Sthenelais zetlandica Thyone roscovita Palaemon serratus Hiatella arctica Glycera tridactyla Ocenebra erinacea Ophiocomina nigra Sthenelais boa Capitella spp. Thyasira flexuosa Cirriformia tentaculata Hinia reticulata Scrupocellaria scruposa Ophiura spp. Modiolarca tumida Leptochiton asellus Ampelisca spinipes Alcyonidium diaphanum Eupolymnia (=Polymnia) nebulosa Hydroides norvegica Lysianassa ceratina Scrupocellaria reptans 2003 Average frequencyof occurrence 1.00 1.00 1.00 1.00 0 0 0 0 1.00 0 1.00 0 0.75 0.25 0.75 0.75 0.25 0.75 0.75 0.75 0 1.00 0 0 1.00 0 0 0 0 2010 Average frequency occurrence 0 0 0 0 1.00 1.00 1.00 1.00 0 0.80 0.20 0.80 0 1.00 0 0 1.00 0 0 0.20 0.60 0.40 0.60 0.60 0.40 0.60 0.60 0.60 0.60 Cumulative (%) 2.04 4.07 6.11 8.14 10.18 12.22 14.25 16.29 20.36 22.05 23.72 25.28 28.36 29.89 31.42 32.95 34.47 35.99 37.50 38.85 40.16 41.37 42.57 43.76 44.95 46.02 47.06 48.16 49.23 98 North east Long Sheelah 2003 vs. 2010 Average dissimilarity = 77.81 Alcyonidium diaphanum Amphipholis squamata Chlamys varia Hinia reticulata Lepidonotus squamatus Maera othonis Modiolus modiolus Mytilus edulis Obelia dichotoma Pododesmus (=Monia) patelliformis Scrupocellaria scruposa Scypha ciliata Sphaerodorum flavum Tubificoides spp. Ascidiella aspersa Mediomastus fragilis Thyasira flexuosa Modiolarca tumida Ocnus brunneus Anomia ephippium Crisia ramosa Sthenelais boa Terebellida spp Boreotrophon truncata Crisia eburnea Hiatella arctica Cellepora pumicosa Chlamys distorta Hymendesmia brondstedi Kirchenpaueria pinnata 2003 2010 Cumulative Average Average (%) frequency of frequency of occurrence occurrence 1 0 1.93 1 0 3.87 1 0 5.80 1 0 7.73 1 0 9.67 0 1 11.60 1 0.25 13.53 1 0 15.47 1 0 17.40 1 0 19.33 1 0 21.27 1 0 23.20 0 1 25.13 1 1 27.07 1 0.20 28.64 1 0.80 30.21 0 0.80 31.77 0.75 0 33.26 0.75 0 34.75 0.75 0 36.21 0.75 0 37.67 0;.75 0 39.13 0.75 0 40.59 0.75 0 42.02 0.75 0 43.48 0.75 0 44.92 0.75 0 46.32 0.75 0 46.32 0.75 0 47.73 0.75 0 49.14 99 Black Rocks 2003 vs. 2010 Average dissimilarity = 77.81 Balanus balanus Calyptraea chinensis Hydroides norvegica Leptasterias muelleri Pholoe synophthalmica Sthenelais zetlandica Caulleriella zetlandica Eteone spp. Melinna palmata Notomastus latericeus Palaemon serratus Pherusa plumosa Scrupocellaria reptans Lucinoma borealis Membranipora membranacea Anomia ephippium Lumbrineris latreilli Semibalanus balanoides Nephtys histricis Venerupis saxatilis Pista cristata Tapes (=Venerupis) rhomboides Euchone southernii Platynereis dumerilii Pomatoceros triqueter Chlamys varia Cirriformia tentaculata Amphipholis squamata Kefersteinia cirrata Apseudes talpa Capitella spp. Terebellida spp. Owenia fusiformis Thyasira flexuosa Sertularia argentea Sagartia elegans Lysianassa ceratina 2003 Average frequency of occurrence 0 0 0 1 0 0 0 0 0 0 0 0 0 0 0 0.20 0 0 0 0 0 0.60 0 0 0 0.60 0.60 1.00 0 0.40 0.60 0.60 0.40 0.40 0.20 0.60 0.40 2010 Average frequency of occurrence 1 1 1 0 1 1 0.80 0.80 0.80 0.80 0.80 0.80 0.80 0.60 0.80 0.80 0.60 0.60 0.60 0.60 0.60 0 0.60 0.60 0.60 0.00 0.00 0.40 0.60 0.80 0 0 0.80 0.80 0.60 0.20 0.80 Cumulative (%) 1.86 3.72 5.58 7.44 9.30 11.16 12.64 141.3 15.62 17.11 18.59 20.08 21.57 23.05 24.53 25.78 26.91 26.91 28.05 31.39 32.50 33.60 34.71 35.81 36.91 38.01 39.11 40.18 41.26 42.33 43.40 44.47 45.33 46.58 47.62 48.66 49.70 100 West Round Island Pinnacle 2003 vs. 2010 Average dissimilarity = 71.51 Antedon bifida Cirriformia tentaculata Lumbrineris latreilli Melinna palmata Pomatoceros lamarckii Semibalanus balanoides Hiatella arctica Sthenelais boa Mya truncata Ampelisca typica Asterias rubens Glycera tridactyla Nephtys histricis Scalibregma celticum Callochiton achatinus Eumida spp. Golfingia vulgaris Notomastus latericeus Scrupocellaria reptans Pista cristata Balanus spp. Membranipora membranacea Myriochele heeri Leptasterias muelleri Nephtys incisa Ophiocomina nigra Lepidonotus squamatus Pododesmus (=Monia) patelliformis Abra alba Ophiothrix fragilis Sthenelais zetlandica Amphipholis squamata Timoclea ovata Hydroides norvegica Cirratulus spp. 2003 Average frequency of occurrence 1 1 0 0 0 0 0.86 0.86 0.86 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0.71 0.71 0.71 1.00 1.00 1.00 1.00 0 0.86 0.43 0 0.57 2010 Average frequency of occurrence 0 0 1 1 1 1 0 0 0 0.80 0.80 0.80 0.80 0.80 0.80 0.80 0.80 0.80 0.80 0.80 0.80 0.80 0.80 0 0 0 0.40 0.40 0.40 0.40 0.60 0.40 1.0 0.60 0 Cumulative (%) 1.80 3.61 5.41 7.22 9.02 10.82 12.38 13..91 15.43 16.93 18.43 19.93 21.43 24.41 25.84 27.82 28.71 30.14 31.55 32.95 32.95 34.32 35.74 37.01 38.27 39.53 40.71 41.88 43.05 44.14 45.23 46.32 47.38 48.43 49.43 101 4.5 In situ observations 4.5.1 North Basin (SS.SBR.SMus.ModCvar) During the surveys in 2003, a small area between Long Sheelah and Hadd Rock was identified as the best example of the Modiolus modiolus with Chlamys varia, biotope (SS.SBR.SMus.ModCvar). The current survey suggests that the biotope in this area has declined in condition (Figure 4.8). There were declines in the abundances of M. modiolus, C. varia, Kirchenpaueri pinnata, and Halecium halecium, between 2003 and 2010. The sponges Spanioplan aramatutram and Iophon hyndmani, which are common on M. modiolus and C. varia shell (B. Picton per obs SLECI) remained absent between 2003 and 2010. There were also no detectable differences in the abundances of the bivalves, Pecten maximus and Ostrea edulis and the sponge M. similaris between 2003 and 2007. Figure 4.8 Photo of M. modiolus communities in the area between south Hadd Rock and north east of Long Sheelah in 2010 4.5.2 South basin (SS.SBR.SMus.Mod.HAs) Surveys in 2003 identified the areas east of Black Rock and West of Round Island Pinnacle as the best examples of the M. modiolus with hydroids and ascidians biotope (SS.SBR.SMus.ModHAs). The M. modiolus communities in these areas 102 showed very little changes between 2003 and 2010 (Figure 4.9). In both areas, there were declines in the density of M. modiolus but increases in the frequency of occurrence of M. modiolus between 2003 and 2010. There were also declines in the abundances of the bivalve C. varia, but very little changes in the abundances of Antedon bifida, Ophiothrix fragilis and Ophiocomina nigra between 2003 and 2010. H. halecinum, C. celata and Pomatoceros triqueter also appear too have increased in abundances in some areas between 2003 and 2010. Figure 4.9 Photo of M. modiolus communities in the area west of Round Island Pinnacle in 2010. 4.5.3 Historical sites within the range of M. modiolus in Strangford Lough The current study also surveyed the areas Green Island, Scott‟s Hole, Jane‟s Rock and Selk Rock, which formed part of the historical range of the M. modiolus communities. In the Scott‟s Hole and Jane‟s Rock areas, M. modiolus and its associated community showed a decrease in abundance between 2003 and 2010. The Green Island area remains unchanged over this period with little or no live M. modiolus but some associated epifauna. The Selk Rock area also remains unchanged with scattered clumps of M. modiolus and some red algae. 103 4.6 Discussion This part of the current study aimed to assess the potential for natural recovery of impacted M. modiolus communities in Strangford Lough by comparing data from MRRG surveys in 2010 with those reported in SLECI (Roberts et al. 2004). Video and photo-transect surveys and quadrat removal sampling of the Modiolus biotope SS.SBR.SMus.ModCvar in the north basin suggest that the abundances and frequency of occurrence of several key species, including Modiolus modiolus itself, the bivalves Chlamys varia, and Aequipecten opercularis and the hydroids Kirchenpaueri pinnata and Halecium halecium , continued to decline between 2003 and 2010. Similar surveys biotopeSS.SBR.SMus.ModHAs in and the quadrat south basin removal sampling demonstrated of significant decreases in the abundances of M. modiolus over the same period. However, the frequency of occurrence of Ophiothrix fragilis and Antedon bifida, Kirchenpaueri pinnata, H. halecium and Ascidiella aspersa were unchanged between 2003 and 2010. These results suggest that many of the characteristic species of the M. modiolus communities, particularly in the north basin, remained absent or in reduced numbers in 2010. The current survey also demonstrated that the diversity of the M. modiolus communities in the north basin had declined relative to surveys in 2003. Video, photo and quadrat surveys of the M. modiolus biotope in the north basin (SS.SBR.SMus.ModCvar) showed that between 2003 and 2010 there were declines in the average density of M. modiolus, the mean number of species, and the Simpson‟s and Pielou indices. In contrast, sampling of the M. modiolus communities in the south basin (SS.SBR.SMus.ModHAs) showed that although there were significant declines in the density of M. modiolus between 2003 and 2010, the mean number of species increased, and there were no clear changes in the Simpson‟s and Pieolu indicesover the same period. These results suggest that despite the total ban on trawling introduced in 2003, M. modiolus communities in the north basin are declining more rapidly than those in the south basin. Using quadrat sampling methods, the current study identified a total of 151 species on M. modiolus communities, with 109 species in the north basin and 117 species in the south basin. The total number of species identified in 2010 was similar to that in the 2003 surveys (Roberts et al. 2004). However, the number of species 104 identified on horse mussel communities in Strangford Lough is lower than total numbers of species on other M. modiolus beds in the UK. Surveys in North Wales, Northern Scotland and the Isle of Man have identified 230 species, 160 species and 270 species, in M. modiolus communities, respectively using removal sampling methods (Mair et al. 2000; Rees et al. 2008; Holt & Shalla unpublished data). The beds at these other sites are classified as a mixture between the SS.SBR.SMus.ModCvar and SS.SBR.SMus.ModHAs communities (Mair et al. 2000; Rees et al. 2008; Holt & Shalla unpublished data) which might explain their higher species richness. 4.7 Conclusions This part of the MRRG project suggests that the M. modiolus communities in the north and south basin remain very much reduced in their characteristic species. The M. modiolus communities in the north basin showed further decline rather than recovery relative to the surveys in 2003. In contrast, the M. modiolus communities in the south basin showed slight recovery in terms of an increase in mean number of species, but showed little changes in species diversity relative to 2003. These results contrast with the previous section (3) which found that, based on photo quadrat sampling, there was a higher diversity of epifauna in the north basin relative to south basin, in the good condition sites. These results are probably explained by the differences in sampling methods and the patchy and fragmented nature of the M. modiolus communities in both the north and south basins. Future sampling should focus on annual, photo quadrat and removal sampling of fixed points in M. modiolus communities in the north and south basins to determine their potential for and rates of natural recovery. 105 5.0 Identifying suitable sites for restoration: habitat suitability modelling for M. modiolus, in Strangford Lough (Undertakings 9 and 10) 5.1 Summary Species restoration requires an understanding of the relationship between species and the environment before conservation programs involving habitat protection, habitat restoration and captive breeding and release, can be fully implemented. This study uses species distribution modelling to identify suitable habitat for the declining marine bivalve, M. modiolus, within Strangford Lough, and provide objective information on sites where intervention and natural recovery will be most successful and likely. Species distribution models were developed for each biotope found within the Lough: the M. modiolus with Chlamys varia, sponges, hydroids and bryozoans (SS.SBR.SMus.ModCvar) in the northern basin and the mixture of M. modiolus with hydroids and large solitary ascidians (SS.SBR.SMus.ModHAs) and M. modiolus with hydroids and red seaweeds (SS.SBR.SMus.ModT) found in the south basin. M. modiolus presence records were collected during SCUBA dive surveys between 2008 and 2010 and environmental parameters from Centre for Environmental Data and Recording (CEDaR) records. Environmental data was interpolated using ARCGIS v9.3 (ESRI, California, USA) to provide environmental layers for distribution modelling using MAXENT, at a common pixel size of 40m. Although substrata importance varied between biotopes, overall M. modiolus distribution was positively associated with the presence of mud and sand and negatively associated with the presence of cobbles, boulders, gravel, bedrock and pebbles. The higher AUC value for the SS.SBR.SMus.ModCvar biotope is most likely an indication of its restricted distribution as opposed to better model fit. 106 Predicted M. modiolus distribution was largely biased towards the centre of the Lough and reflects the known historic distribution. 107 5.2 Introduction Subtle changes in the environment are highly influential in determining an animal‟s distribution (Strayer 2008) and understanding the relationships between an animal and its habitat is required when attempting to restore a declining species. Using a tool that gives an understanding of the variation of the quality of an organism‟s habitat enables the prioritization of areas for conservation (e.g. Wilson et al. 2011) and is important in reducing effort and cost required to manage rare or threatened species (Olsson & Rogers 2009). Habitat suitability mapping is frequently used to identify areas in need of restoration or preservation (Gibson et al. 2004), or identify candidate areas in species reintroduction programs (Olsson & Rogers 2009). Predictive species-specific landscape favourability models, based on Geographic Information Systems (GIS), have become the favoured method in defining species habitat requirements (Guisan & Zimmerman 2000; Wilson et al. 2011). For example, Wilson et al. (2011) employed Species Distribution Modelling (SDM) using the opensource software MAXENT to identify population augmentation and reintroduction sites within rivers in Northern Ireland for the globally endangered freshwater bivalve, Margaritifera margaritifera (L.). In biogeographical studies it is generally considered that a species is most abundant in the centre of its range (Hochburg & Ives 1999), and therefore in the centre of the environmental range of variables which it occurs. This is the „abundant centre‟ distribution model (Sagarin & Gaines 2002). Although it may be argued that predictive distribution studies based on a declining species may be biased if they are developed when a species is no longer found throughout its historic range, they should still indicate key elements of its niche. This is because chronic changes in environmental conditions are likely to have a greater impact on those individuals at the edge of the range than those in the centre (Wilson et al. 2011). Conservation measures to enhance the recovery of a species after a period of decline should first involve total protection followed by habitat restoration. However, captive breeding and release is frequently used as a major conservation strategy, but is in reality the option of last resort (Wilson & Roberts 2011). There are concerns that releasing captive-bred animals into the wild is less successful than translocating 108 individuals from other wild, healthy populations (Griffith et al. 1989; Wolf et al. 1996). Nonetheless, a clear understanding of the species-specific habitat niche allows the identification of potential release sites with the maximum chance of post-release survival, often demonstrating a need for habitat restoration prior to reintroduction. Consequently conservationists are increasingly emphasizing the integration of distribution models with reintroduction and population augmentation programs (Seddon et al. 2007; Sergio et al. 2007). M. modiolus beds are classified as three biotopes within Strangford Lough: the M. modiolus with Chlamys varia, sponges, hydroids and bryozoans (SS.SBR.SMus.ModCvar) in the northern basin and the mixture of M. modiolus with hydroids and large solitary ascidians (SS.SBR.SMus.ModHAs) and M. modiolus with hydroids and red seaweeds (SS.SBR.SMus.ModT) found in the south basin. M. modiolus has declined throughout the Lough since the 1970s (Magorrian & Service 1998; Service 1998; Section 2, this report). A key element of the current project was to develop intervention methods to arrest this decline (Section 6). These methods, based on experience gained in major oyster restoration projects in the USA (Mann & Evans 2004; Schulte et al. 2009) include translocation of healthy individuals from wild populations to unmodified sites or sites where shell cultch has been laid, or the release of captive-bred animals. This part of the current project was therefore undertaken to identify sites where such intervention techniques are likely to have greatest chance of success. The aim of this section (undertakings 9 & 10), was to develop predictive distributional maps using habitat favourability models based on interpolated environmental variables for M. modiolus in different biotopes and throughout Strangford Lough. These maps will identify areas of high conservation value for each basin which can be used to select non-disturbance areas and sites for restoration involving the use of cultch, captive-bred or translocated mussels (Section 6). 109 5.3 Methods 5.3.1 Landscape parameterization During the current project M. modiolus was recorded at 124 locations in Strangford Lough by ROV and SCUBA diving surveys conducted between 2008 and 2010 (Figure 5.1). Environmental parameters, as percentage cover of substrata, have been documented by CEDaR and are shown in Table 5.1. Depth data was excluded from the analysis because of the potential for bias to sampling areas within diving and ROV depth limits, which would exclude deeper areas of the Lough. Although many historical records exist for M. modiolus in Strangford Lough, it was decided to limit the species presence records to the most up-to-date distributional data because its presence may influence substrata composition. Consequently, because the loss of M. modiolus may cause a change in substratum characteristics, using historical data may be less biological relevant to current conditions within the Lough and the selection of restoration sites. Point data on substratum type and percentage cover, derived from SCUBA diving, containing habitat variables were interpolated using the Inverse Distance Weighted (IDW) interpolation tool in the Spatial Analysis toolbox in ARCGIS v9.3 (ESRI, California, USA). ArcGIS v9.3 was used to extract habitat variables on a landscape scale resampled to a common pixel size of 40m throughout Strangford Lough. Table 5.1 Substrata recorded during SCUBA diving and used in MAXENT model. Variable name Unit Description Bedrock Boulders Cobbles Mud Pebbles Sand % % % % % % Coverage of bedrock derived from interpolated dive data Coverage of boulders derived from interpolated dive data Coverage of cobbles derived from interpolated dive data Coverage of mud derived from interpolated dive data Coverage of pebbles derived from interpolated dive data Coverage of sand derived from interpolated dive data 110 Figure 5.1 Locations of M. modiolus biotopes in Strangford Lough, Northern Ireland, UK. Purple shaded areas represent estimated historical distribution of SS.SBR.SMus.ModCvar (Chlamys varia) biotope; green shaded areas represent estimated historical distribution of SS.SBR.SMus.ModHAs / SS.SBR.SMus.ModT (Ophiothrix sp.) biotopes (Connor et al. 2004). Blue dots represent M. modiolus records found in SS.SBR.SMus.ModCvar (n = 46), red dots represent M. modiolus records found in SS.SBR.SMus.ModHAs / SS.SBR.SMus.ModT biotopes (n = 51) during MRRG dive surveys, used in MAXENT modelling. 111 112 5.3.2 Statistical analyses MAXENT 3.2.1a (Phillips et al. 2006; Phillips & Dudlík 2008) was used to predict the probability of species occurrence at a pixel size of 40 m. Due to the restricted range of M. modiolus it was hypothesised that the species‟ habitat associations would occupy a narrow band of tolerance and, therefore, not display linear relationships. Consequently, model flexibility was maximised by considering quadratic, product, threshold, hinged and discrete functions for all habitat parameters (Phillips & Dudlík 2008). Jack-knife resampling analysis was used to determine a heuristic estimate of the relative contribution of each variable based on the performance of the global model (known as test gain) without the variable of interest compared to the influence of that variable in isolation (derived from a univariate model only). Global model performance was judged using the area under the curve (AUC) in the receiver operating characteristic (ROC) analysis (Liu et al. 2005). Model significance was tested using a one-tailed binomial test of omission (the fraction of test occurrence falling outside the prediction) under the null hypothesis of random prediction, given the same fractional predicted area. Marginal response curves of the predicted probability of species occurrence were graphed for each explanatory variable that contributed substantially to the global model. A map of habitat favourability for M. modiolus was generated to reflect the predicted probability of species occurrence using ARCGIS v9.3. Because of limitations on species presence records the one-tailed binomial test of omission was only conducted for the model containing both biotope records. Model testing was also carried out for MAXENT model containing species presence records for both biotopes using a test set of 25% of presence records. The fit of the model to the test data is the real test of the model‟s predictive power using the AUC value. In the original tender contract, other variables were to be included into the habitat suitability model as predictor variables for M. modiolus distribution modelling. However, depth was not included in the model because dive depths and ROV sampling for M. modiolus did not occur deeper than approximately 40m, so there is the possibility of sampling bias. 113 5.4 Results 5.4.1 SS.SBR.SMus.ModCvar The occurrence of M. modiolus in SS.SBR.SMus.ModCvar biotope was negatively associated with cobbles, gravel, pebbles, bedrock and boulders, explaining 42.6%, 10.1%, 9.6%, 8.7% and 8.4% of the variation in distribution, respectively (Figure 5.2a). The probability of M. modiolus occurrence was virtually zero where the percentage cover of these coarser substrata was high (Figure 5.3a). However, M. modiolus presence was more or less positively associated with mud and sand substrata explaining 9.1% and 7.8% of the variation in distribution, respectively, with the probability of species occurrence close to 1 when the percentage cover of finer substrata close to 100% (Figure 5.2a & 5.3a). Model performance, defined as the area under the curve (AUC), was highly discriminative with AUC = 0.98, indicating that M. modiolus in this biotope occupied a highly specific habitat niche, in terms of substrata. Habitat favourability is largely biased towards the western side of the mid region of Strangford Lough (Figure 5.4a). 5.4.2 SS.SBR.SMus.ModHAs/ModT Occurrence of M. modiolus in biotope SS.SBR.SMus.ModHAs/ModT was negatively associated with boulders, bedrock, gravel, pebbles and cobble, explaining 31.7%, 20.3%, 10.5%, 8.6% and 7.7% of the variation in distribution, respectively (Figure 5.2b). The probability of M. modiolus occurrence was virtually zero where the percentage cover of these coarser substrata was high (Figure 5.3b). However, M. modiolus presence was more or less positively associated with sand and mud substrata explaining 12.1% and 9.2% of the variation in distribution, respectively, with the probability of species occurrence close to 1 when the percentage cover of finer substrata close to 100% (Figure 5.2b & 5.3b). Model performance, defined as the area under the curve, was discriminative with AUC = 0.87. Habitat favourability is largely biased towards the mid region of Strangford Lough (Figure 5.4b). 114 5.4.3 Both biotopes Overall, the occurrence of M. modiolus was negatively associated with cobbles, boulders, gravel, bedrock and pebbles, explaining 30.9%, 18.9%, 14.6%, 9.6% and 4.7% of the variation in distribution, respectively (Figure 5.2c). The probability of M. modiolus occurrence was virtually zero where the percentage cover of these coarser substrata was high (Figure 5.2c & 5.2b). However, M. modiolus presence was more or less positively associated with mud and sand substrata explaining 13% and 8.2% of the variation in distribution, respectively, with the probability of species occurrence close to 1 when the percentage cover of finer substrata close to 100% (Figure 5.2c & 5.2b). Habitat favourability is largely biased towards the mid region of Strangford Lough (Figure 5.4c). The one-tailed binomial test was significant (P < 0.01), indicating the model successfully predicts occurrence records significantly better than random (Anderson et al. 2003). Model performance, defined as the area under the curve, was highly discriminative with AUC = 0.92. Test AUC was 0.83 ± 0.052 (standard deviation) indicating a good model fit. 115 a) Chlamys b) Ophiothrix c) Both biotopes Cobbles (42.6) Boulders (31.7) Cobbles (30.9) Gravel (10.1) Bedrock (20.3) Boulders (18.9) Pebbles (9.6) Sand (12.1) Gravel (14.6) Mud (9.1) Gravel (10.5) Mud (13) Bedrock (8.7) Mud (9.2) Bedrock (9.6) Boulders (8.4) Pebbles (8.6) Sand (8.2) Sand (7.8) Cobbles (7.7) Pebbles (4.7) 0.0 0.5 1.0 1.5 % test gain 2.0 2.5 0.0 0.2 0.4 0.6 0.8 1.0 % test gain 1.2 1.4 1.6 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 % test gain Figure 5.2 Jackknife analyses of the importance of environmental variables in maximum entropy modelling of M. modiolus occurrence throughout Strangford Lough in a) SS.SBR.SMus.ModCvar biotope, b) SS.SBR.SMus.ModHAs/ModT and c) both biotopes. A heuristic estimate of the relative contribution of each variable to the global model is given in parentheses whilst variables are listed in descending order of importance. Grey bars show the performance of the global model (known as test gain) without each variable and black bars show the influence of each variable in isolation (derived from a univariate model only). 116 a) b) c) Figure 5.3 Marginal response curves of the predicted probability of M. modiolus occurrence throughout Strangford Lough in: a) SS.SBR.SMus.ModCvar biotope, b) SS.SBR.SMus.ModHAs/ModT biotope and c) both biotopes for explanatory variables that contributed substantially to the global maximum entropy model. The x-axis represents percentage cover for each substratum type; the y-axis represents probability of species occurrence. 117 a) b) b) c) Figure 5.4 Biogeographical models of habitat favourability for M. modiolus in a) SS.SBR.SMus.ModCvar biotope, b) SS.SBR.SMus.ModHAs/ModT biotope and c) in both biotopes throughout Strangford Lough, providing a means to identify areas of high conservation value for the species. 118 5.5 Discussion This study aimed to provide objective information for conservation management about sites where intervention, i.e. translocation or captive breeding and release, and natural recovery is potentially most likely for M. modiolus within Strangford Lough. This is the first study to examine the relationships between M. modiolus and substrata suitability. Currently, the most favourable habitat for M. modiolus is in the mid region of Strangford Lough. M. modiolus distribution is quite restricted with the probability of occurrence peaking within narrow bands of the spectrum of variability available within most environmental parameters. Although the model revealed slight differences in the relative importance of different substrata in explaining the distribution of each biotope, in general M. modiolus was positively associated with softer finer substrata and negatively associated with coarse substrata (Figure 5.3). The model predicted that the probability of occurrence of M. modiolus, was very high where softer finer substrata, such as mud and sand, predominated and virtually zero in areas dominated by coarser substrata, such as cobbles, boulders, gravel and bedrock. Overall, the distribution of M. modiolus predicted by the model reflected the known historic distribution (Section 2). The model was also able to identify discrete areas well outside the current distribution as being suitable for M. modiolus. Chiefly, the northern end of „The Narrows‟, the north, east and south west perimeter of the distribution model, and the western perimeter of the Strangford Lough basin. The relationship between M. modiolus and substrata can be explained by wave exposure, current and sedimentation. The entrance to Strangford Lough has a high current speed during ebb and flow tides. Because of current speed in this region, there is a lower likelihood of sedimentation. That is, M modiolus spat would be less likely to fall and settle in areas with greater tidal or current velocity. Therefore, the positive association observed with sand and mud may reflect areas of the Lough that permits spat to settle on the sea bed. A higher model AUC value for the SS.SBR.SMus.ModCvar (northern) biotope than the SS.SBR.SMus.ModHAs/ModT southern biotopes indicates a much narrower habitat niche, in terms of substrata. As substrata are most likely highly collinear with current speed and sedimentation, we feel that they are robust variables. Time did not permit the inclusion of other 119 variables held by AFBI, such as Chlorophyll α (Chl α); the potential for the inclusion of such data should be investigated further. The habitat suitability map supports the proposal for a total protection zone within the mid region of the Lough (Section 8) and will inform the selection of potential restoration sites (Section 6.1). 120 6.0 Intervention actions The long term objective of the Modiolus reef Restoration Plan (MRP) is “to restore the Strangford Lough Modiolus biogenic reef feature to Favourable Conservation Status”. If natural recovery is not observed, active restoration approaches are needed. The main aim of this part of the project was to investigate and develop intervention methodologies to restore M. modiolus reefs in Strangford Lough. The specific methodologies tested by the Modiolus Restoration Research Group (MRRG) are based on restoration protocols and guidelines for other bivalve species (Caddy & Defeo 2003; Brumbaugh et al. 2006). This section of the report deals with the following intervention approaches: 6.1 Translocation of natural M. modiolus reefs (Undertaking 11). 6.2 Provision of substrate to create favourable sites and enhance natural recruitment (Undertaking 12). 6.3 Culturing M. modiolus in a dedicated hatchery, re-seed beds and monitor recovery (Undertaking 13). 121 6.1 Translocation clumped adult or restructuring Modiolus modiolus of scattered, and un- subsequent monitoring (Undertaking 11) 6.1.1 Summary The Modiolus Restoration Plan requires development of methodologies to restore the Modiolus modiolus reef feature in the event of natural recovery not being observed. Translocation of adult M. modiolus into degraded sites is one of the options tested to assess if M. modiolus biotic conditions improve. An artificial reef was constructed using king scallop, Pecten maximus, as cultch for 6000 relaid adult M. modiolus to determine survival of translocated mussels, as well as assess the effect of elevation. The present experiment was conducted within the southern basin of Strangford Lough where the M. modiolus community typically belongs to a mixture of M. modiolus with hydroids and large solitary ascidians (SS.SBR.SMus.ModHAs) and M. modiolus with hydroids and red seaweeds (SS.SBR.SMus.ModT) biotopes . The experimental design incorporated elevated and flattened artificial reefs, and mussels relaid directly on the seabed. Survival in all treatments was high and no significant difference in mortality occurred between treatments. Numbers of species associated with the constructed reef increased with greater habitat complexity of the reef structure. This was interpreted as a reflection of the natural reef forming process by M. modiolus. 122 Spat collectors deployed near the cultch site indicate natural recruitment of M. modiolus spat from sources in the surrounding area. Regular monitoring of the artificial M. modiolus reef is advised. The monitoring should include adult mussel survival, reef elevation and structure, natural M.modiolus spat recruitment and diversity of the associated reef community. This experiment has demonstrated that translocation of adult horse mussels onto purpose-built artificial reefs consisting of shell cultch is likely to enhance recovery and natural recruitment providing additional brood-stock to damaged areas. It is recommended that a similar trial is conducted at a suitable site in the northern basin to stimulate recovery of the SS.SBR.SMus.ModCvar biotope. 123 6.1.2 Introduction Translocation of shellfish stocks to enhance natural populations in depleted or damaged areas is known as restocking (Caddy & Defeo 2003). Restocking has been used to enhance natural shellfish populations of scallops (Peterson et al. 1996), oysters (Mann & Evans 2004) and clams (Rice et al. 2000). Restocking activities often involve and may require a combination of habitat manipulation (addition of settlement substratum or „cultch‟) and hatchery production in order to restore the population (Caddy & Defeo 2003). Loss of relief (elevation above the seabed) is a common feature of all damaged biogenic reefs (Schulte et al. 2009) and as a result, construction of 3-dimensional reefs has become a widely used approach, particularly in the USA. These elevated reefs also enhance the recruitment and survival of, for example, oysters and their associated reef communities providing interstitial space within the reef matrix and increasing the survival of spat (Bartol & Mann 1997). Few, if any of the remaining M. modiolus beds in Strangford Lough are elevated. In addition, many of the remaining animals are in scattered clumps. The loss of elevation has several important repercussions for the reef which include 1) the reduction in the water flow around the reef and hence food supply, 2) greater sedimentation on the reef via re-suspension from the seabed and natural settlement of particles, 3) increased disease dynamics and 4) reduced dispersal of gametes and larvae because of reduced water flow. Some of the effects are probably habitatspecific, but all studies to date indicate enhanced bivalve performance with increased elevation above the seabed as a result of increased food flux, reduced sedimentation and/or reduced low oxygen stress (Schulte et al. 2009). The deployment of cultch has proved highly successful in the regeneration of biogenic reefs formed by different bivalve species worldwide (Uttting 1988; Héral 1990; Leard et al. 1999; Wesson et al. 1999; Cranfield et al. 2001; Smyth 2007). Within Strangford Lough, intervention based on concentrating scattered M. modiolus into viable reef clumps will need to assess the required relief yet minimise the translocation of locally collected cultch and live M. modiolus. As a result of these requirements a medium-scale experiment was designed involving the deployment of cultch at one site South East of the Brown Rocks (Figure 6.1.1). It is hypothesised that the deployment of this shell cultch will create a more suitable area for the 124 translocation of wild adult and hatchery reared M. modiolus. The cultch deployed will also increase the availability of natural settlement substratum for wild M. modiolus spat thus enhancing natural recruitment. The artificial reefs will also increase habitat complexity therefore increasing the biodiversity in the associated faunal assemblages (Cranfield et al. 2004). 6.1.3 Aims and objectives The overall aims of this part of the current project were to develop field techniques to deploy cultch at experimental sites and to evaluate the use of cultch as a longterm strategy for the restoration of M. modiolus biotopes in Strangford Lough. Specific objectives were to: 1) Determine if survival and expansion of translocated clumped M. modiolus are enhanced on elevated cultch plots when compared to flattened cultch and unmodified substratum. 2) Establish baseline data for future investigations into biological community change through succession that may result from deployment of cultch. 3) Investigate the natural settlement of M. modiolus spat on cultch plots. 6.1.4 Materials and Methods 6.1.4.1 Site selection The main considerations to select a suitable site for shellfish restoration include: 1) the proposed site must be within the historic distribution range of the species in question; 2) seabed condition must be suitable to support the addition of shell cultch and translocated stocks; 3) the area should ideally be a „sink‟ area for larvae transported from a „source‟ area; 4) currents should be sufficient to deliver food and oxygenated water; and 5) avoid areas where threats to shellfish stocks still exist (Brumbaugh et al. 2006). Two cultch deployment sites within the historic distribution range of M. modiolus in Strangford Lough were initially proposed 1) South East of Sand Rock (54° 125 27.9468‟N; 5° 33.9396‟W) and 2) East of Brown Rocks (54° 25.5382‟ N, 5° 36.7194‟W) (Figure 6.1.1). Baseline surveys were carried out by divers to assess whether the site selection considerations above were met. The proposed site South of Sand Rock consisted of very fine sediments with active burrows of Norwegian Prawn Nephrops norvegicus. This seabed type was considered too muddy for M. modiolus to find any attachment points and survive. The second site, East of Brown Rocks was characterised by strong currents of up to 4 knots at mid-tide. It was considered that the strong currents at this site precluded safe deployment of the cultch and would have disturbed the experiment before the translocated mussels successfully attached to the cultch. Both sites were deemed unsuitable for cultch deployment and restocking experiments. An alternative third location South of Brown Rocks was surveyed and found suitable (section 6.2.3 below). Cultch deployment was approved by DARD Fisheries division and NIEA after the mandatory public consultation period and a FEPA Marine Construction Licence and a Section 14 Fisheries Permit were acquired.. The selected cultch deployment site was situated South of Brown Rocks (540 25‟18.9”N – 050 37‟14.94”W). The proposed area extends 20 m to the north and south of these points and the total area is 27 m 2 (Figure 6.1.1). 126 Figure 6.1.1 MRRG proposed and amended cultch deployment sites. 6.1.4.2 Survey methodology A buoyed shot line was dropped in the centre of the site. Two transects were surveyed from the north east to the south west and from the south east to the north west of the proposed sites. Four spot searches using a circular search pattern were also carried out at 10 m and 20 m intervals (Figure 6.1.2). Photographs were taken every 10 m along transects (Figure 6.1.3) and species occurrence and abundances were recorded (Table 6.1.1). 127 Transect lines Circular survey lines Centre of surveyed areas Figure 6.1.2 Schematic of survey strategy carried out at potential cultch deployment sites. 6.1.4.3 Site selection survey results The third proposed site was located within the South Basin M. modiolus distribution range. M. modiolus beds have been mapped by the MRRG in close proximity to the site. Seabed composition consisted of fine sand with overlying broken mixed shell (mostly M. modiolus), with few pebbles and cobbles (Figure 6.1.3). This substrate type is characteristic of the sublittoral biotopes SS.SBR.SMus.ModHAs/ModT (Connor et al. 2004) found in Strangford Lough‟s South Basin. The faunal assemblage observed is typical for this substratum type and it is not regarded as rare. Phyla recorded included sponges, cnidarians, tunicates, bryozoans and echinoderms. The tunicate Ascidiella aspersa and the crinoid Antedon bifida were the most frequently recorded species (Table 6.1.1). The proposed site is also considered a „sink‟ for M. modiolus larvae as spat collector and population structure analysis (Section 6.2) documented high recruitment levels in the area. The location South of Brown Rocks met the main pre-requisites to attempt the cultch deployment and translocation experiment. 128 Figure 6.1.3 Seabed surface at the cultch site area pre-cultch deployment. The tunicate Ascidiella aspersa and the burrowing anemone Cerianthus lloydii (left photograph) were some of the species recorded. Table 6.1.1 Species associated with cultch site South of Brown Rocks (*SACFOR scale abundance codes) Species Abundance* Suberites carnosus Cerianthus lloydii Antedon bifida Echinus esculentus Asterias rubens Ophiocomina nigra Ophiura albida Flustra foliacea Ascidiella aspersa O C F O F F O O F 6.1.4.4 Artificial reef experimental design Four randomly allocated plots were established for each treatment: elevated, flattened and „off-cultch‟ (Figure 6.1.4). 129 Figure 6.1.4 Position of randomly allocated cultch plots south of Brown Rocks. 6.1.4.5 Deployment of cultch Approximately 10 tonnes of weathered scallop shell were sourced from a local shellfish processing plant at Portavogie, Co. Down. Aging of the shells used in shellfish restoration programs is recommended to avoid transmission of parasites or pathogens (Bushek et al. 2004). The shell was bagged into 0.5 tonne lots using polypropylene sacks for transportation and ease of deployment. A Manitou © crane was used to load the bagged shell onto two 11 x 6 m barges (Figure 6.1.5a). The deployment barge was equipped with a HiAb© crane and when on-site, a 1 tonne mooring block was lowered to act as anchor for the support vessels and attachment point for a Ultra Short Baseline (USBL) acoustic beacon and Odyssey® data loggers (Figure 6.1.6). Once on-site the deployment barge used the crane to lower the first bag to a depth of 1m below the surface to allow trapped air to escape from the bagged shell (Figure 6.1.5b). Once the bag was negatively buoyant it was un-hitched from the crane and lowered to the seabed on a buoyed shackled deployment rope. Using a strap and shackle the second bag was attached to the deployment rope. When negatively 130 buoyant, the strap was released and the bag was sent down the rope. This process was repeated until all the remaining bags for the plot were in position. This procedure was carried out at the seven remaining sample stations until all 10 tonnes were on-site. Once the bagged shell was in-situ and the barges were off-station, divers working in teams of two descended the buoyed deployment ropes (Figure 6.1.5 c & d) to connect the cultch deposits using guide lines. The deployment ropes were unshackled by the divers thereby leaving no obvious sign of the sample plots on the surface. The bagged shell was left on the seabed for 1 month to ensure it was firmly in place, allowing any air pockets trapped between the scallop valves time to disperse from the sacks. Divers working in teams of two descended a shot line, using the guide line to find the individual plots. The sacks were removed using a rope and lifting strap which was passed through all four of the lifting loops (Figure 6.1.7). Diver 1 remained in-situ on the top of the sack while Diver 2 cut three sides. The divers then ascended with the rope. Once the divers were out of the water, the rope was made fast and the sack was dragged clear and retrieved. The procedure was repeated at the seven remaining sample stations. The plots at each sample station were prepared by the divers to match the four elevated and four flat configurations of the experimental design. The divers flattened 4 of the cultch plots and built up the 4 elevated plots to a height of approximately 2m. All experimental plots were marked sublittorally with a numbered flag (Figure 6.1.8). The plots were then left for a further two weeks to allow the cultch to stabilise before translocation of M. modiolus commenced. 131 a) b) c) d) C) d) Figure 6.1.5 Cultch deployment procedures: a) Unloading bagged shell from Manitou© crane and b) HIAB® bag transfer to deployment barge, c) dive team prepares to enter water, d) divers descend deployment rope. a) a) b) 132 Crane Deployment barge Bagged shell Figure 6.1.6 Deployment of bagged shell from anchored barges. Lifting strap leading to surface Attached sack loops Cut sides Figure 6.1.7 Sack retrieval and shell deployment. 6.1.4.6 Translocation of adult Modiolus modiolus One of the options considered at the beginning of the project was to source ca. 800 kg of adult M. modiolus from the beds located in Donaghadee Sound for deployment in suitable areas in Strangford Lough. The use of non-local populations in shellfish restocking programs is not recommended as the newly introduced population might not adapt to the local environmental conditions (Caddy et al. 2003). The use of local broodstock also minimizes the risk of introducing alien species that 133 can adversely affect the local communities. Therefore the M. modiolus stocks used in the translocation experiments were collected from Strangford Lough populations. The MRRG dive team carried out collection dives on two fragmented beds located near the cultch six weeks prior to the cultch deployment. This resulted in a total translocation sample of approximately 6000 M. modiolus. The sample was bagged into polypropylene mesh sacks at 40 individual mussels per sack and left in running seawater tanks in Queen‟s University Marine Laboratory for over two months in preparation for deployment. Once the cultch plots were positioned, the mesh sacks were deposited on the seabed and attached to a mooring block where they remained for 4 weeks. Previous observations from Roberts et al. (2004) suggested that mortality among adult M. modiolus by Asterias rubens will be significantly reduced in a clumped structure. This procedure maximised byssal attachment between the individual mussels allowing the formation of tightly bound clumps. Once the cultch plots were stabilised and the M. modiolus had formed clumps, ca.500 mussels were placed on each experimental treatment (Figure 6.1.8). 6.1.4.7 Monitoring In-situ environmental monitoring began after translocation of broodstock mussels concluded on the second week of March 2010. Parameters recorded included bottom temperature and irradiance by means of data loggers attached to the mooring block. Odyssey® data-loggers were set to record data every 10 minutes from March to November. Current profiling was carried out on the second week of May while chlorophyll levels were monitored monthly from May to November 2010. Spat collectors were also deployed nearby to monitor natural spat recruitment levels. Replicate design and natural recruitment results at the experimental cultch site are described in section 6.2. A baseline survey was carried out in April 2010 using digital video and still photography. The survey documented the structural conditions of the experimental plots immediately after deployment also documenting any marine fauna attracted to the site. Divers returned to the cultch experiment in September 2010. One diver photographed replicate 0.0625m2 quadrats in each treatment plot while the second 134 diver carried out a video survey of the area. The photographs and video footage were analysed in the laboratory to document mussel survival and epifaunal recolonization. Figure 6.1.8 Raised cultch experimental plots one month after M. modiolus translocation April 2010. 135 6.1.4.8 Additional clumping behaviour experiment Clumping in M. modiolus was studied in a field experiment using different quantities of dead shell to assess if viable clumps can be generated using fewer mussels. Live M. modiolus were mixed with 25%, 50 and 75% M. modiolus shells and placed in biodegradable bags (Table 6.1.2). Divers randomly positioned a total 28 bags in a location within the Scott‟s Hole site leased to Queen‟s University Belfast by the Crown Estates. The location proved unsuitable as high mortalities occur probably due to the soft muddy sand substrate and current regimes that characterises it. Table 6.1.2 Treatments for the re-clumping experiment. Treatment Live M. modiolus/shell mixture 100% 66% 33% 0% Replicates per site 7 7 7 7 M. modiolus per bag 45 30 15 0 Shell per bag 0 30 60 90 315 630 210 630 105 630 0 630 Total treatment M. modiolus Total Modiolus for a site 6.1.5 Results 6.1.5.1 Epifaunal community succession 6.1.5.1.1 Baseline survey A survey was carried out 1 month after the experimental lay-out was completed and 2 weeks after the all M .modiolus had been translocated and relaid on all treatments. Opportunistic taxa such as echinoderms and crustaceans were common on the plots and adjacent area. The starfish Asterias rubens and the sea urchin Echinus esculentus were common on the flattened and unmodified substratum plots 136 but absent from the raised plots. Other echinoderm species included the starfish, Henricia oculata, and the sun star, Crossaster paposus. Crustaceans common in all plot types included the velvet swimming crab, Necora puber, the hermit crab, Pagurus bernhardus, and the brown crab, Cancer pagurus (in order of abundance). The spider crab, Macropodia rostrata, and the green crab, Carcinus maenas, were also present. Fish species were attracted to the artificial reefs early after deployment. The most commonly recorded species were the two-spotted goby, Gobiusculus flavescens and goldsinny wrasse, Ctenolabrus rupestris. Benthic fish species such as the black goby, Gobius niger and the butterfish, Pholis gunnellus, were also recorded among the scallop shells and in the crevices left between the sacks. 6.1.5.1.2 Follow-up 6-month survey There was a dramatic increase in the numbers and abundances of species documented by divers during the survey carried out 6 months after the start of the translocation experiment. Mobile species appeared to be using the artificial reef for food and refuge while sessile species such as hydroids and tunicates had colonized the entire substrate, making mussel mortality counts difficult. A total 39 different species belonging to 8 different phyla were recorded during the the monitoring dives and included 7 Porifera, 4 Cnidaria, 1 Polychaeta, 9 Crustacea, 7 Echinodermata, 1 Tunicata, 2 Bryozoa and 7 Osteychties (Bony Fish) (Table 6.1.3; Figures 6.1.10-12). 137 Table 6.1.3 Presence/absence list of associated fauna. Cultch site, South Brown Rocks Phylum Species name Pre-deployment Baseline survey April 2010 Post-deployment survey September 2010 PORIFERA Halichondria panicea 0 0 1 Suberites carnosus 1 0 0 Halecium spp 0 0 1 Abietinaria abetina 0 0 1 Nemertesia antennina 0 0 1 Obelia spp. 0 0 1 POLYCHAETA Pomatoceros spp. 0 1 1 CRUSTACEA Balanus balanus 1 0 1 Balanus crenatus 1 0 1 Liocarcinus depurator 0 0 1 Cancer pagurus 0 0 1 Carcinus maenas 0 1 1 Macropodia spp. 0 0 1 Inachus dorsettensis. 0 0 1 Necora puber 0 0 1 Pagurus bernhardus 0 1 0 Macropodia rostrata 0 1 0 Homarus gammarus 0 0 1 Antedon bifida 1 0 1 Ophiocomina nigra 1 0 1 Ophiura albida 1 0 1 Asterias rubens 0 0 1 Crossaster papposus 1 1 1 Echinus esculentus 0 0 1 Henricia oculata 0 1 0 TUNICATA Ascidiella aspersa 1 1 1 BRYOZOA Scrupocellaria spp. 0 1 1 Flustra foliacea 1 1 1 Ctenolabrus rupestris 0 1 1 Phollis gunnelus 0 1 1 Gobius niger 0 1 1 Callionymus lyra 0 0 1 Gobiusculus flavescens 0 1 1 Gadus morhua 0 0 1 CNIDARIA ECHINODERMATA VERTEBRATA (PISCES) 138 Total number of species per taxon 10 9 8 Porifera 7 Cnidaria 6 Polychaeta 5 Crustacea 4 Echinodermata 3 Tunicata 2 Bryozoa 1 Pisces 0 Baseline 3 months 6 months Figure 6.1.10 Chart showing the clear increase in marine life diversity since the deployment of the artificial M. modiolus reef in the South Brown Rocks area in March 2010. Mean number of species ±SD 12 10 8 6 4 2 0 Baseline 3 months 6 months Figure 6.1.11 Chart showing the temporal changes in biodiversity of the faunal assemblage associated with the cultch plots 139 September 2009: Site selection survey • Seabed consists of shelly sand • Ascidians and burrowing anemones dominate the community • Sparse mobile epifauna (starfish, sea urchins) March 2010: Cultch deployment and translocation of M.modiolus completed April 2010: Baseline survey (after M. modiolus translocation) • Sea urchins, starfish and crabs immediately attracted to the experimental site • Fish species including wrasse, gobies and butterfish using the plots for refuge and food September 2010: Follow-up monitoring survey • • • • • Ascidiella aspersa colonises the substrate Other epiphytes such as hydroids, bryozoans and sponges are recorded for the first time Sea urchins, starfish and other opportunistic mobile epifauna are common in all treatments Fish species abundant Crabs and lobster present Figure 6.1.12 Community change associated with the artificial M. modiolus reefs. 140 6.1.5.2 Effect of relief on M. modiolus survival Individual M. modiolus clumped fast and remained attached to the substratum and the cultch structure was intact 6 months after deployment. Divers initially suggested that elevated plots offered more protection against mobile predators such as starfish, Asterias rubens, green crab, Carcinus maenas and sea urchin, Ecinus esculentus, which were common on the flattened cultch plots and untreated substratum plots. Mobile scavengers including some A. rubens feeding on M. modiolus were recorded in all treatments during the 6-month follow-up survey. However, survival rates were high in all treatments (Figure 6.1.13): 1) Raised=92%; 2) Flattened=79%; and 3) Substratum= 82%. Overall, mortalities of translocated mussels were slightly higher on untreated substratum and flattened cultch plots but survival was not significantly better on elevated cultch plots (ANOVA: F(1,24)=0.831; p>0.05). Figure 6.1.13 Graph showing differences in M. modiolus survival in each treatment 6 months after deployment (± standard deviations). 141 6.1.6 Discussion Substratum type and hydrodynamic conditions at the artificial M. modiolus reef site were initially regarded as suitable and the results obtained during the baseline and follow-up surveys confirmed the experiment had been established successfully. Mussel survival was also high in all treatments with no significant advantages from reef elevation. The natural elevation of M. modiolus reefs in relatively undamaged conditions, such as the Craigyouran and Round Island Pinnacle beds, is not higher than 50 cm (MRRG divers, pers. obs.). It is possible that reef elevation is not critical in a species reported to range from epifaunal (Davenport & Kjørsvik 1982) to semiinfaunal (Meadows & Shand 1989) or infaunal (Holt et al. 1998) in its life habits. It is too soon to reject the hypothesis that substratum elevation provides significantly better survival conditions for M. modiolus and further surveys will be necessary. The translocated mussels rapidly attached themselves to the scallop shell and formed tight clumps, replicating their natural clumping behaviour. The constructed reef attracted a large number of mobile, largely opportunistic species soon after deployment. Sessile epifauna re-colonized the shell surfaces of the translocated mussels; hydroids, bryozoans and sponges usually associated with horse mussel beds were observed by divers during the follow-up survey six months after deployment. Pseudofaeces and sediment accumulated in the spaces between the mussel clumps and the scallop shell increasing habitat complexity and attracting polychaetes, amphipods and other small macroinvertebrates. Habitat complexity increases the niches available for different species in the habitat recolonization process. Cranfield et al. (2004) reported differences in the macrofaunal assemblage of sites of different levels of habitat complexities in the Foveaux Straits, New Zealand, after oyster dredging operations ceased. The range of habitats included badly impacted reef areas and localized regenerated patches in the dredged seafloor. Cranfield et al. (2004) postulated a model of macrofaunal succession of increasing complexity during the habitat regeneration process. The first stage after impact included re-colonization by bivalves and encrusting bryozoans followed by mussels, Modiolus areolatus, and oysters, Ostrea chilensis, which attracted gastropods and served as settlement substrata for tunicates and mussel and oyster spat. As provision of suitable substrata increases so does the complexity of the 142 community with polychaetes, sponges and bryozoans forming the last stages of the biogenic reef and the assemblage succession (Cranfield et al. 2004). The assemblage succession recorded in the brief period between the completion of the reef construction and the first monitoring survey six months later probably reflects the natural reef forming process by M. modiolus communities. All the species recorded are common in the SS.SBR.SMus.ModHAs/ModT biotope typical of Strangford Lough‟s South Basin. In any shellfish restoration program the concept of larval sources and sinks for stock replenishment is important. Source populations have frequent recruitment and a good representation of different size classes (Caddy et al. 2003). Natural recruitment studies were carried out by the MRRG and the results are described in Section 6.2. (Figures 6.1.7 and 6.1.8). Size and age-frequency histograms were bimodal with over 50% of the population consisting of sizes less than 20mm or mussels less than 10 years of age indicating a high degree of larvae settlement in the South Basin area. The other age class consisted of adult mussels of ages ranging from 20 to 45 years. Spat collectors positioned within the artificial reef from April to July 2010 also yielded some M. modiolus spat (Section 6.2; Figure 6.1.10), suggesting natural recruitment in the area adjacent to the cultch is occurring. The horse mussel beds in close proximity to the experimental M. modiolus reef (Brown Rocks, Selk Rock) are potential „sources‟ of M. modiolus spat. However, alternative sources of larvae may include areas outside Strangford Lough as the south basin experiences greater water exchange with the Irish Sea (Boyd 1973). This question would best be addressed by a hydrodynamic study involving particle tracking modelling and population genetics. It will also be necessary to carry out small-scale removal sampling to assess if the translocated mussels are also enhancing natural recruitment to the experimental M. modiolus reef. Work to develop a full larval dispersal model specifically tailored for M. modiolus in Strangford Lough is well underway. The model incorporates the hydrodynamic effects of the islands and pladdies while adding larval development data obtained during the pilot aquaculture trials (section 6.3). When completed, the model could be used to select optimal sites for restoration involving cultch deployment and translocation of M. modiolus in future. 143 6.1.7 Conclusions The cultch deployment and M. modiolus translocation experiment was an operational success. Monitoring to date revealed no significant differences in M. modiolus survival between elevated cultch, flattened cultch and untreated substratum. The results from the follow-up monitoring survey were also very positive. Soon after deployment there were signs of a natural M. modiolus succession underway. The increased habitat complexity attracted numerous species to an otherwise barren area within the historic range of the species. There were also high survival rates in the translocated mussels which rapidly clumped together. The potential for natural recruitment enhancement needs to be tested but preliminary spat collection experiments and population structure data shows abundant supply of larvae from M. modiolus beds. The substratum experiments in section 6.2 indicate that settlement of M. modiolus larvae is directly enhanced by the presence of adults on the seafloor therefore a combination of cultch provision and broodstock translocation, similar to the described pilot M. modiolus reef constructed by the MRRG, is the recommended restoration approach. 144 6.2 Provision of suitable substrata for spat settlement and subsequent monitoring (Undertaking 12) 6.2.1 Summary The Modiolus Restoration Plan intervention action proposed the use of suitable substrate to enhance natural recruitment. The Modiolus Restoration Research Group (MRRG) implemented the intervention action by investigating recruitment patterns of Modiolus modiolus in several locations representative of its distributional range in Strangford Lough. Natural recruitment was very poor in damaged areas north of the Long Sheelah (SS.SBR.SMus.ModCvar biotope). Natural rescruitment was very high in the Southern distribution range (SS.SBR.SMus.ModHAs/ModT biotope), which is probably self-sustaining. Substratum experiments confirmed settlement rarely occurs outside the matrix created by live adult M. modiolus, Settlement was significantly better among clumps of live mussels. Spat settlement was very poor on artificial spat collectors and loose M. modiolus and Pecten maximus shells. The use of artificial blue mussel spat collectors for cultivation of M. modiolus is not a viable restoration approach. 145 6.2.2 Introduction The second intervention action element of the Modiolus Reef Restoration Plan involves testing sites within Strangford Lough that may be favourable to Modiolus modiolus spat settlement by deploying artificial hard substrata (shell cultch) and spat collectors. Provision of additional artificial substrata to promote natural recruitment is a key element in shellfish restoration programs (Beck et al. 2009). For example, placement of shell or shell fragments increases recruitment of hard clams Mercenaria mercenaria (Kraeuter 2003) and oysters Crassostrea virginea (Powers et al. 2009). This section investigates ways to increase the provision of such substrata. This undertaking has two experimental sections: 1) Investigation of M. modiolus spat recruitment. Data on larval fluctuation will be gathered using spat collectors and population structure information at different locations representative of the two distinct M. modiolus biotopes found in Strangford Lough. 2) Studies on the quality of the cultch as refugia for settled M. modiolus spat The aim was to produce an analysis of the settlement and recruitment data recorded along with an analysis and the costs and benefits of collecting M. modiolus spat in the context of restoration. 6.2.3 Materials and methods Population structure and recruitment results were compared between the two basins within Strangford Lough (Connor et al. 2004; Roberts et al. 2004). These biotopes are: 1) “Modiolus modiolus beds with Chlamys varia, sponges, hydroids and bryozoans on slightly tide-swept very sheltered circalittoral substrata (SS.SBR.SMus.ModCvar)”, recorded exclusively in the North Basin; 2) the mixture of M. modiolus with hydroids and large solitary ascidians (SS.SBR.SMus.ModHAs) and M. modiolus with hydroids and red seaweeds (SS.SBR.SMus.ModT) which is 146 recorded mainly in the South Basin, although there is an overlap into the North Basin (Ringhaddy Sound, Craigyouran). 6.2.3.1 Natural recruitment Natural recruitment of M. modiolus spat was recorded by means of population structure sampling and purposely built spat collectors. Adult horse mussels were collected from 6 different locations representative of the two M. modiolus biotopes present in Strangford Lough (Figure 6.2.1). The SS.SBR.SMus.ModCvar biotope present north of the Long Sheelah is in poor condition and very reduced in extension (Roberts et al. 2004; MRRG surveys), therefore sampling was limited to just two locations, South of Hadd Rock and North West of Long Sheelah. The SS.SBR.SMus.ModHAs/ModT biotope is more widespread and the extent and condition is not considered as heavily damaged as theSS.SBR.SMus.ModCvar biotope. A total of 4 different locations representative of this biotope were sampled: South of Craigyouran (new M. modiolus bed found by the MRRG in 2008), West of Round Island Pinnacle, East of Black Rock and off Selk Rock. Samples consisting of 150 mussels from each population were collected by divers using randomly positioned 0.25m2 area quadrats. The contents from each quadrat were removed in its entirety and placed into 1mm mesh bags. Upon return to the laboratory all M. modiolus clumps were carefully checked for spat. All spat (<20mm) was graded into different sizes using a set of metallic sieves of different mesh size. Adult and young mussels (>20mm) were counted and their length, height and width measured to the nearest millimetre for use in population structure analyses. 6.2.3.2 Shell aging methods A total of 30 mussels representative of the size range were selected from the population structure samples to study growth rate and age structure for each biotope. In the laboratory all mussels were cleaned of epifauna, the M. modiolus spat (<20 mm) was counted, measured and the winter bands counted to estimate the age. The soft tissues from horse mussels bigger than 20 mm in length were removed 147 and the shells were dried for 2 hours at 60°C in an oven. Small shells (<40mm) were directly embedded in Metaset® resin manufactured by Buehler Ltd., UK and sectioned using a circular saw after the resin had solidified. Larger shells were cut prior to embedding. Once the resin was fully dried the embedded shells were ground and polished using wet and dry sandpaper of different sizes. The shell surfaces were finally etched using 0.01M HCL for 20 minutes. Acetate peel replicas of the sectioned shells were obtained following the process described by Richardson et al. (1979). When viewed under the microscope, the peels reflect the growth lines present in the inner and middle nacreous shell layers, the latter more clearly visible. The darker growth lines represent winter growth and can be counted to give an age estimate of each mussel (Anwar et al. 1990). Figure 6.2.1 Subtidal samples consisting of ca.150 where collected at 6 locations representative of the two M. modiolus biotopes found in Strangford Lough, A) SS.SBR.SMus.ModCvar: Hadd Rock and Long Sheela; B) SS.SBR.SMus.ModHAs/ModT: Selk Rock, Black Rock, Craigyouran and Round Island Pinnacle. 148 6.2.3.3 Spat settlement Three different treatments were tested for use in spat collection and substratum preference experiments: king scallop Pecten Maximus shell, M. modiolus shell, and live adult M. modiolus. A tray without contents was included as a control. The weight/volume/surface area of the cultch was standardised before deployment. Each treatment was set up in an oyster tray (51cm x 51cm) and the four trays were joined together with cable ties (Figure 6.3.3). In addition, scrubbing pads and two types of commercially available mussel spat collecting materials used in blue mussel (Mytilus edulis) cultivation were deployed simultaneously on the same rigs: Christmas rope and Swedish band (Figure 6.2.4). A 50 cm length of each spat-collector material was attached to the cultch unit. The experiment was replicated five times; each experimental rig was pegged out to the seafloor at 3 experimental sites: Hadd Rock, Scott‟s Hole and Brown Rocks (Figure 6.2.2). These units remained in place from June 2009 to October 2010. Supplementary spat collectors where deployed near the artificial M. modiolus reef constructed by the MRRG Southwest of the Brown Rocks (Section 4.1). The spat collectors consisted of monofilament net and M. modiolus shells joined by rubber bands, to simulate the surfaces where M. modiolus spat are usually found in natural conditions (M. modiolus byssus threads and empty bivalve shells and hydroids and periostracal hairs). The collectors were replicated 5 times, placed inside mesh bags and positioned inside sections of plastic pipes of 15 cm diameter (Figure 6.2.5). The pipes served as protection against scavengers and also helped to fix the spat collectors to the seabed at the same time. The spat collectors were deployed in April 2010 and retrieved in August 2010. 149 Figure 6.2.2 Map showing the location of the cultch units and spat collectors deployed by the MRRG in both the SS.SBR.SMus.ModCvar and SS.SBR.SMus.ModHAs/ModT biotopes (north and south basins respectively). 150 MARKER BUOY SPAT COLLECTOR: X-MAS TREE ROPE Scallop shell Live Modiolus modiolus SPAT COLLECTOR: 50 cm SWEDISH BAND Modiolus modiolus shell Control 51 cm 51 cm OYSTER TRAYS Figure 6.2.3 Diagram of the cultch units used in the recruitment experiments. MRRG, November 2008. 151 1) 2) Figure 6.2.4 Spat collectors used in the experiment: 1) Swedish band and 2) Christmas tree rope. Figure 6.2.5 Spat collectors placed on the seabed near the experimental M. modiolus reef. Pipe tubing was used to protect the experiment from predators. 152 On return to the laboratory all spat collectors and cultch experiments were submerged in 5% Sodium Hypochlorite to separate the mussels (Beduschi et al. 2009) and carefully checked for M. modiolus spat under a stereomicroscope. All M. modiolus juveniles were counted and the attached epifauna identified, counted, fixed in 4% Formaldehyde and finally preserved in 70% Ethanol. Two-way ANOVA analysis with replication was carried out to compare recruitment in each treatment and identify between the different locations studied. 6.2.4 Results 6.2.4.1 Population structure and natural recruitment Population structure of M. modiolus is represented by a bimodal size frequency distribution, particularly distinctive in the SS.SBR.SMus.ModHAs/ModT biotope populations (Figure 6.2.6). Peaks of abundance for SS.SBR.SMus.ModCvar (Hadd Rock sampling station) are 5 to 10mm and 80 to 85mm; Mode = 80.7; Mean ± SD = 80 ± 7.3 max. length = 100 mm. Medium size mussels between 20 and 60 mm were absent from the sampled population. In the SS.SBR.SMus.ModHAs/ModT biotope (represented by the Round Island Pinnacle M. modiolus bed) the peaks are 1 to 5 mm and 85 to 90 mm; Mode = 86.7; Mean ± SD = 86.7 ± 11.06; max length = 162mm. Medium size adults between 20 and 60 mm were found but in very low numbers representing just 3% of the total sample population. The M. modiolus population sampled in 2003 by Roberts et al. (2004) was similar, with slightly smaller modal peaks of length between 70 and 80mm in the North Basin (SS.SBR.SMus.ModCvar) populations and 75 to 80 mm in the South Basin (SS.SBR.SMus.ModHAs/ModT). In 2003, the largest mussel from North of the Long Sheelah was 96.3 mm in length while the largest from the Southern Basin (Black Rock) was 113.5 mm, all smaller than the mussels recorded during the sampling carried out by the MRRG in 2009 and 2010. 153 45 40 35 Frequency (%) 30 25 20 SS.SBR.SMus.ModCvar (N=341) 15 SS.SBR.SMus.ModHAS (N=1391) 10 5 0 Shell Length (mm) Figure 6.2.6 Length-frequency histograms in 5mm groupings of M. modiolus collected from the two biotopes recorded in Strangford Lough: In blue SS.SBR.SMus.ModCvar (poor quality M. modiolus beds, North Basin) and in red SS.SBR.SMus.ModHAs/ModT (undamaged beds, South Basin). Samples were randomly collected by divers in 2009 and 2010. Recruitment was higher in the South Basin SS.SBR.SMus.ModHAs/ModT biotope, where M.modiolus spat represented 53% of the total 1391 mussels sampled. The SS.SBR.SMus.ModCvar M. modiolus community by contrast was clearly dominated by older individuals with seed horse mussels accounting for less than 5% of the total sample (N=341). Natural recruitment levels are displayed in Table 6.2.1 and Figure 6.2.7. 154 Table 6.2.1 Modiolus modiolus spat (Length <20 mm) frequencies recorded from 6 sites in Strangford Lough during June 2009 and 2010 Long Sheelah and Hadd Rock represent the SS.SBR.SMus.ModCvar biotope (North Basin) while the remaining stations are all within the SS.SBR.SMus.ModHAs/ModT biotope (South Basin) Modiolus population Long Sheela Hadd Rock Craigyouran Round Island Pinnacle Black Rock Holm Bay Adults 176 154 261 167 107 113 Spat 10 7 205 Total 186 161 466 Recruitment 5.38% 4.35% 43.99% 175 203 161 342 310 274 51.17% 65.48% 58.76% Figure 6.2.7 Comparative chart of natural recruitment levels of M. modiolus spat sampled from 6 distinct beds in Strangford Lough. Colours represent biotopes: blue= SS.SBR.SMus.ModCvar; red= SS.SBR.SMus.ModHAs/ModT. 155 6.2.4.2 Growth and age-frequency distributions Estimated growth rates were compared using shell age estimation data recorded from samples representative of each biotope (SS.SBR.SMus.ModCvar: N = 30; SS.SBR.SMus.ModHAs/ModT: N = 71). The length at age data was fitted to the von Bertalanffy growth equation using estimation methods described by King (1995). The von Bertalanffy equation in terms of length is Lt = L∞ (1 – e–kt) where L∞ is the length at age t, L∞ is the theoretical maximum length the population of M. modiolus will reach if it lived indefinitely and k is the measure of the growth rate. The parameters L∞ and k were estimated by the Ford-Walford method (King 2003). TheSS.SBR.SMus.ModCvar population sampled had the highest growth rate and lower maximum predicted shell length (k = 0.082 and L∞ = 90.51 mm) whereas mussels from theSS.SBR.SMus.ModHAs/ModTbiotope reached larger sizes at a slower rate (k = 0.074; L∞ = 116.2 mm). 140 Shell Length (mm) 120 100 80 60 40 20 0 0 10 20 30 40 50 60 Age Figure 6.2.8 Estimated Von Bertalanffy growth curves for M. modiolus from theSS.SBR.SMus.ModCvar (blue) andSS.SBR.SMus.ModHAs/ModT (red) biotopes. Data points represent mean length calculated for each age value. 156 Young mussels were recorded in both biotopes indicating recruitment occurs in both albeit at very different frequencies. The age-frequency distributions show also a bi-modal distribution. The SS.SBR.SMus.ModCvar biotope shows <10% of the total population are young mussels (<5 years) while the percentage in the southern SS.SBR.SMus.ModHAs/ModTbiotope in as high as 60%. The overall trend for older mussels in both populations is to have a majority of mussels aged 20-30 years old. The oldest mussels in the SS.SBR.SMus.ModCvar community were 40 years old. The age distribution in the SS.SBR.SMus.ModHAs/ModT community is more spread with a higher proportion of older specimens of 40 and 45 years of age. 70 Frequency (%) 60 50 40 30 SS.SBR.Smus.ModCvar 20 SS.SBR.Smus.ModHAS 10 0 5 10 15 20 25 30 35 40 45 Age (yr) Figure 6.2.9 Age-frequency histograms for M. modiolus collected from each biotope. Blue=North Basin‟s M. modiolus and C. varia biotope (N = 62); red = South Basin‟s M. modiolus with hydroids and ascidians (N = 294) 6.2.4.3 Spat settlement Artificial spat collectors attached to the cultch experimental trays yielded no results for M. modiolus spat. Settlement of M. modiolus spat on the monofilament and joined M. modiolus shell collectors was low when compared to the cultch experiments (Figures 6.2.10 and 6.2.12). A total 35 spat less than 1cm in length were recovered from the monofilament net treatments while only 13 were found on horse mussel shells. Statistical analysis of the results showed monofilament net was 157 significantly more effective in attracting settlement of M. modiolus pediveligers (t-test, p<0.05). Figure 6.2.10 Comparison of M. modiolus spat recruitment using monofilament net and M. modiolus shells after a 4-month period. September 2010. Figure 6.2.11 Photograph showing 4 months old M.modiolus spat (2 mm long) collected using monofilament net. 158 6.2.4.4 Spat settlement One year after deployment all trays were removed and the spat and associated fauna counted and preserved. Natural settlement of M. modiolus spat was significantly higher in the South Basin live-clump treatments while it was almost negligible for both the North Basin and the remaining treatments (Figure 6.2.12). Presence of M. modiolus spat was limited almost exclusively to the clumps of live M. modiolus (F(3,32) = 11.87; p < 0.001). The differences in settlement of M. modiolus were noticeable between the South and North Basins. After one year left on the seabed the live clumps from the South Basin trays yielded significantly higher numbers of M. modiolus spat than the mussels from the North Basin experiments (F(1,32) = 12.1; p < 0.01). Figure 6.2.12 Histogram showing substrate preference results as mean abundance of Modiolus modiolus spat per treatment and geographical location. 159 6.2.5 Discussion 6.2.5.1 Population structure and natural recruitment In Strangford Lough previous studies described a M. modiolus population dominated by mature individuals where spatfall was usually poor. Mussels less than 20mm accounted for a small proportion of the population (Seed & Brown 1975; Roberts et al. 2004). The size-frequency histograms showed the bi-modal distribution typical of the species with the highest peak of abundance around 90mm shell length. M. modiolus with similar population structurehave also been reported from some parts of Scotland; poor recruitment rates puts the viability of such M. modiolus populations into question (Comely 1978). Comely (1981) also recorded few if any spat from samples collected in Shetland voes. This bi-modal population structure is believed to reflect the „escape through growth‟ life history strategy of M. modiolus. Young mussels are subjected to intense predation when young so energy is initially directed to growth but once individuals reach a size that is too large to be eaten by their major predators (mostly crabs) they re-direct energy to reproduction (Roberts 1975; Seed & Brown 1978). Population structure analysis carried out in 2009 and 2010 by the MRRG show natural recruitment of M. modiolus occurring in Strangford Lough with significant differences between the 2 different biotopes. The results are comparable to those from 2004 as regards to presence of M. modiolus spat (<20mm) from sampling sites north of the Long Sheelah (ModCvar biotope). Here seed mussel accounts for only 5% of the total population. The M. modiolus and C. varia biotope SS.SBR.SMus.ModCvar therefore is damaged not only in quality and extent but it may no longer be self sustaining anymore. The histograms from populations south of the Long Sheelah (ModHAS biotope) show spat frequencies of almost 70% in some locations (Craigyouran, Round Island Pinnacle). Recruitment from these beds is not only much higher than in the SS.SBR.SMus.ModCvar biotope but it is also substantially higher than the recruitment rates reported from the same beds in 2004 (Roberts et al. 2004); at this time spat only accounted for 20% of the Black Rock population and it was less than 5% of the M. modiolus population sampled west of Round Island Pinnacle. This increase in recruitment since 2004 could be a result of recent spatfall events 160 although monthly sampling carried out from 2008 to 2010 showed consistent high spat frequencies in clumps collected from the SS.SBR.SMus.ModHAs/ModT biotope. The medium sizes (20 to theSS.SBR.SMus.ModHAs/ModT 60mm) are histogram clearly but not underrepresented absent like in in theSS.SBR.SMus.ModCvar populations indicating that the population might be selfsustaining with enough animals reaching the „refuge‟ size of 40-50mm (Roberts 1975) In shell-length frequency distributions for short-lived species like Mytilus edulis each modal peak usually indicates different year classes (Seed 1969). In slow growing and long lived bivalves each size group is a combination of different year classes, particularly in the bigger mussels. The smaller size classes may contain just a proportion of young mussels, as has been reported for the freshwater pearl mussel Margaritifera margaritifera (Hastie et al. 2010). The age frequency distribution data obtained from the shell-aging data correlates to the size-frequency histograms with two distinct groups found in each population. Young mussels (<10 years of age) still represent a very small fraction of the damaged SS.SBR.SMus.ModCvar biotope while the majority of the population consists of old adult mussels between 20 and 45 years of age. In theSS.SBR.SMus.ModHAs/ModTbiotope some adults from the bigger size class are younger than expected from the size-frequency histogram therefore it is clear that a fraction of young mussels is underrepresented in the sizefrequency data. Larval dispersal models are currently being developed by Queen‟s University Belfast oceanographers and engineers. This hydrodynamic model includes tridimensional aspects of the current regimes within Strangford Lough providing vital information on dispersal behaviour to supplement the natural recruitment findings. It is suspected that current regimens along with the decrease in size of the source population in the North Basin are partly responsible for the low recruitment rates observed by the MRRG during the 2009-10 periods. 6.2.5.2 Substratum preference The results obtained from spat collectors were poor for M. modiolus spat. Previous use of artificial spat collectors to record natural recruitment (Roberts et al. 2004) also yielded very low numbers of M. modiolus spat after a few months. 161 Considering the very high spatfall levels recorded from wild clump samples and live mussel clumps during the tray experiments it is evident that spat collectors grossly underestimate natural recruitment. Cultivation of the blue mussel Mytilus edulis is based in the collection and growing of naturally settled spat (Galley et al. 2010). The collection of naturally settled Modiolus modiolus spat using artificial blue mussel spat collectors was also tested to ascertain if enough seed could be collected for transferring to out-growing aquaculture facilities. As spat was not naturally attracted to the artificial spat collectors placed on the experimental trays this approach is discounted as a viable option for collection of M .modiolus seed for restoration. Horse mussels are gregarious bivalves, forming clumps that rise above the seabed providing refuge not only to a diverse array of infaunal and epifaunal species but also to their own juveniles. M.modiolus spat prefer to settle among conspecifics; successful recruitment or spat survival seldom occurs outside the microhabitat created by the adult live M.modiolus (Rees 2005; MRRG observations). The results obtained from the substrate experiments also showed a highly significant preference to settle on live adult mussels. This behaviour suggests that deployment of inert cultch as a method to enhance natural recruitment is not a sufficient method if not supplemented with translocated mussels. The presence of live adult mussel appears to be crucial to triggering settlement in the horse mussel larvae. It is worth pointing out that, although poor when compared with live clumps, settlement on monofilament net was higher than on other materials. This may reflect the preference of M. modiolus pediveligers to settle primarily on filamentous substrata such as hydroids commonly recorded on the shells of live adult M. modiolus to later migrate to the interstitial spaces. These associations of recently settled mussels with filamentous substrata have been reviewed by Bayne (1965) and Seed (1969). Mytilus edulis plantigrades are known for settling on hydroids and algae and not directly onto the mussel beds. 162 6.3 Pilot Modiolus modiolus hatchery cultivation (Undertaking 13) 6.3.1 Summary The Modiolus reef Restoration Plan contemplates the production of young M. modiolus for experimental reseeding if natural recruitment is not observed. To meet this objective the Modiolus Restoration Research Group (MRRG) set up a dedicated M. modiolus hatchery. Horse mussel spat was successfully produced from local broodstock. Conditioning was not necessary. Partial desiccation effectively triggered spawning in broodstock mussels. The full larval cycle from fertilized eggs to settled pediveligers takes approximately 38 days at ambient summer water temperatures for Strangford Lough. No significant differences were observed in larval survival and growth using single or mixed algal diets. Pediveligers preferred settling among live mussels than on artificial substrata. 1.5mm spat were obtained after four months in an upwelling system. The main obstacles to produce enough quantities of spat for reseeding were: 1) lengthy developmental cycle; 2) slow larval and spat growth; 3) poor survival rates; and 4) very specific settlement requirements The high costs associated with running the hatchery operations compared to the poor return in seed means hatchery production of M. modiolus is not a viable restoration option at this stage. 163 6.3.2 Introduction The use of hatchery produced M. modiolus spat to enhance natural recruitment is one of the intervention action elements listed in the restoration plan. Successful hatchery production of M. modiolus seed requires an in-depth knowledge of the reproductive and larval cycle (Roberts et al. 2004) but there has been little effort to cultivate M .modiolus because of its low commercial value. The breeding cycle of M. modiolus varies between populations within its distribution range (Brown 1984). Spawning may occur annually (Comely 1978; Brown 1984; Jasim & Brand 1989; Kaufman 1977 in Flyachinskaya & Naumov 2003) or intermittently with intervals of up to 5 years between spawning events (Lilleskare 1905; Wiborg 1946; Rowell 1967). In Strangford Lough the reproductive cycle of M. modiolus appears to be different than other populations. Brown and Seed (1977) concluded that the reproductive cycle in the Strangford Lough subtidal M. modiolus population lacks any seasonality. During this 3-year study Brown and Seed (1977) reported a constant presence of both ripe mussels and settled spat therefore concluding that the broodstock population releases a trickle of gametes throughout the year. There are few published examples of spawning induction of M. modiolus in the laboratory are rare (Williamson 1907; Schweinitz & Lutz 1976; Jasim 1986). Jørgensen (1946) measured the eggs of M. modiolus and described and compared larval morphology to Mytilus edulis. Rees (1950) concluded that the smallest veligers of M .modiolus and M. edulis are similar in shape although the former has a less pointed narrow end. Schweinitz & Lutz (1976) described for the first time the full larval cycle of M. modiolus from fertilized egg to pediveliger larva using broodstock from Maine (USA). The first pediveligers were recorded 19 days from fertilization while the larvae remained in the water column for about a month. Flyachinskaya & Naumov (2003) were not successful in triggering spawning in aquarium-kept M. modiolus broodstock from the White Sea but obtained fertilized eggs after spontaneous spawning events and documented all larval stages from fertilized egg to metamorphosed pediveliger. Unfortunately, the appearance and duration of each embryonic phase was not reported. 164 The pilot hatchery set up by the Modiolus Restoration Research Group aimed to produce large numbers of settled spat for use in experimental reseeding trials. Objectives were 1) induce spawning of broodstock M. modiolus collected from Strangford Lough; 2) obtain viable eggs and fertilize them; 3) grow the larvae to pediveliger stage documenting the developmental cycle; and 4) obtain settled spat for use in the experimental re-seeding. 6.3.3 Materials and Methods To produce M. modiolus spat the MRRG set up an M. modiolus hatchery using Queen‟s University Belfast Marine Laboratory aquaculture facilities. The facility has a constant reliable supply of good quality sea water, algal culturing rooms and is not far from the M. modiolus beds used to provide the broodstock used in the experiments. The pilot M. modiolus hatchery followed standard bivalve mollusc hatchery techniques (Helm et al. 2004): 1) Production of micro-algae for provision of food; 2) Conditioning brood-stock; 3) Induction of spawning and husbandry of larvae to settlement; 4) Intermediate cultivation (Figure 6.3.1). 165 Figure 6.3.1 M. modiolus hatchery operation scheme (adapted from Utting & Spencer 1991) 6.3.3.1 Production of micro-algae. Four different algal species were tested as food for the M. modiolus larvae: the diatom Chaetoceros calcitrans; and the flagellates Nannochloris atomus, Isochrysis galbana (clone T-Iso) and Tetraselmis suecica. A batch culture system was used to rear the species to adequate quantities (Figure 6.3.2). Algae stocks obtained from Queen‟s University Marine Laboratory and the Scottish Association of Marine Science (SAMS) algal culturing facilities in Oban (Scotland) were used to inoculate 500 mL starter cultures. The starter cultures were used to inoculate scaled-up cultures containing sterilized seawater diluted with distilled freshwater and added F/2 media (Helm et al. 2004 for composition) and silica (Si). The cultures were aerated to provide a carbon source for photosynthesis and constantly illuminated by a battery of fluorescent lights (Helm et al. 2004). Once algae grew to the point where cell density inhibited light penetration (judged by colour) cultures were scaled-up to intermediate cultures of 3, 5, and 10 L in volume. Once the 10 L cultures reached critical density they were transferred to 100 L polyethylene bags. These 100 L cultures were used 166 as food for the M. modiolus larvae (Figure 6.3.2). Due to their small cell sizes, C. calcitrans, N. atomus and I. galbana are most suitable for the early veliger stages of M. modiolus while the bigger cells of T. suecica can only be assimilated by pediveligers and juveniles. In 2008 mixed results growing microalgae meant that only C. calcitrans and T. suecica could be used, because cultures of I. galbana T-ISO repeatedly crashed before reaching harvestable volumes. In 2009 all three algal species were satisfactorily grown in enough quantities to feed the M. modiolus larvae during the embryonic and settlement phases. The success in the production of live algae allowed the set up of an experiment to find the best feeding regime. A total of six different algal diet combinations were used to test the effect on mean size, survival and development of the larvae (Table 6.3.1). N. atomus was the only algal species grown in sufficient quantities to guarantee a steady supply during the 2010 trials. A single-species diet using N. atomus was tested with positive results. Some studies report successful results when partially substituting live algae diets with microencapsulated diets (Laing 1987). The use of commercially available microencapsulated dry diet supplement (MySpat® manufactured by INVE Ltd.) was also planned in an effort to reduce the quantities of cultured microalgae needed in the hatchery. Figure 6.3.2 Microalgae batch culturing facilities at Queen‟s University Marine Laboratory, Portaferry. Left microalgal stock cultures and right, 100 L culture bags. 167 6.3.3.2 Conditioning of brood-stock and induction of broodstock spawning The M. modiolus broodstock used in the pilot hatchery was sourced from Strangford Lough North and South Basin populations. The mussels were cleaned of epifauna to minimise contamination of the gametes and held in tanks with a constant supply of running sea water (running to waste and not re-circulated) at ambient temperatures (between 13°C and 15°C). Broodstock subsamples were taken to assess gonad ripeness. Both male and female specimens had full or near full gonads in all cases therefore the conditioning phase was skipped. These observations on gonad ripeness match those of Seed & Brown (1977). Spawning induction is the method in which mature bivalves are induced to liberate their gametes in response to applied stimuli (Utting & Spencer 1991). Induction methods tested included: 1. Thermal cycling. Broodstock was transferred to shallow trays (Figure 6.3.3) and submerged in heated sea water at 25 °C for 30 to 40 minutes with added microalgae to induce filtration activity. The warm water was replaced by running sea water at ambient temperature (15 °C) for one hour. The cycle was repeated four times. 2. Addition of gametes. Some mussels were also dissected to check gonad ripeness. These gonads were added to two of the conditioning tanks in the 2008 experiments. During the 2009 hatchery work sperm and ova was added to the water if spawning was unsuccessful after the 3 rd warm-cold water cycle. The gametes were released close to the inhalant siphons using a Pasteur pipette (Figure 6.3.3) 3. Air exposure. In 2008 a spontaneous spawning event was recorded after the holding tanks were accidentally drained overnight. A controlled air exposure treatment was used during the 2009 and 2010 trials (Figure 6.3.3). Spawning was recorded in all events in less than 24 hours after refilling the spawning trays. 168 All broodstock was returned to the collection areas in Strangford Lough once spawning was achieved. Figure 6.3.3 Spawning trays with M. modiolus broodstock under spawning induction treatments: being subjected to air exposure (left) and addition of gametes (right). 6.3.3.3 Larval husbandry Male and female broodstock were not kept separately and fertilization occurred in the holding tanks after spawning. All fertilized oocytes and early larval stages already formed were gently washed into a plastic beaker using UV filtered seawater through a 45 μm sieve. Subsamples were taken and total numbers of fertilized eggs estimated using a Coulter counter. Representative samples were taken and preserved in 4% formaldehyde. The beaker contents were placed in separate 85 L semiconical PVC tanks filled with UV filtered sea water and constantly aerated at a low flow rate to avoid damage (Figure 6.3.4). Cultures were diluted to achieve densities of 10 to 15 larvae mL-1 to avoid overcrowding. 169 Figure 6.3.4 Modiolus modiolus spawning trays (left) and larval rearing containers (center) Water was changed every second day by siphoning it out the holding tanks into a stack of sieves of different aperture mesh, starting with a sieve of 300µm to retain most of the grit and followed by sieves of increasing mesh size as larval development progressed. Any sieved fraction that contained mostly detritus and dead larvae was discarded. A sample of the healthy larvae was retained and the larvae counted. M. modiolus larval development was monitored by sampling 50 veligers. Information recorded included: numbers of each larval stages present, general appearance of the larvae, mortality rates, shell dimensions as well as representative light photomicrographs. The sample was preserved in 4% Formaldehyde and the remaining larvae were returned to the rearing vessel. Larvae were fed every day with different volumes of cultured microalgae calculated using the formula: Required cell density (cells/μL) x V c 1000 Volume (mL) = Cell density of harvested algae (cells/μL) (Utting & Spencer, 1991). Grazed algae densities were calculated between each water change and fresh algae added to restore algal densities. The effect of single and mixed diets on larvae survival and growth was also tested. The results for each 170 treatment were subjected to a repeated measures ANOVA to identify significant differences. The microalgal species and species combinations used and their concentrations are listed in Table 6.3.1. Table 6.3.1 Microalgal feeding regimes used in the M. modiolus hatchery. 2008-10 (following concentration guidelines by Helm et al. 2004) Year Treatment Species Proportion Concentration (%) (cells/µl) 2008 - C. calcitrans 100 250 2009 1 C. calcitrans 100 250 2009 2 I. galbana T-ISO 100 50 2009 3 C. calcitrans + I. galbana T- 50/50 125/50 ISO 2009 4 C. calcitrans + T. suecica 50/50 125/5 2009 5 I.galbana T-ISO +T.suecica 50/50 50/12.5 2009 6 C.calcitrans + I. galbana + T. 33/33/33 83/33/3.3 suecica 2010 - N. atomus 100 250 When larvae reached pediveliger stage water was re-circulated in an upwelling system and changed every second day checking for presence of swimming veligers remaining in the water column. Larvae were fed daily, adding 50% more food every passing week. Different settlement materials were tested including scrubbing pads, monofilament net, live adult mussels and empty M. modiolus shells. Variables measured included settlement material preference and settled larval growth and survival. 171 6.3.3.4 Intermediate cultivation Intermediate cultivation consists of maintaining the spat in a nursery system until they reach a sufficient size to resist the effect of predation and competition in the wild (Gosling 2003). Any settled spat obtained from the previous phase was placed in a down-welling recirculation system, normally used for species sensitive for overcrowding such as clams and scallops (Helm et al. 2004). This was a more conservative approach as the adequate relaying densities for M. modiolus pediveligers are not known. Food calculations followed the formula: F = (S X 0.4)/7 where F is the dry weight of algae (N. atomus, weight= 21.4 pg/cell) required per day in mg and S is the live weight of spat at the beginning of each week (after Utting & Spencer 1991). 6.3.4 Results 6.3.4.1 Broodstock size frequency Broodstock collected from the South Basin M. modiolus beds had a mean length of 98 mm and a length modal peak of 100 mm while the largest mussel was 124 mm long. Males accounted for 55% of the total sample. The samples from Hadd Rock (North Basin) had a modal peak length of 84 mm, an average length of 77 mm while the largest mussel was 97 mm in length. Males accounted for 66% of the total broodstock sample. The size frequency histograms (Figures 6.3.5a and 6.3.5b) omit the spat present within the clumps. 172 Hadd Rock Horse Mussels a 30 Frequency/no units 25 20 15 10 5 0 50.054.9 55.059.9 60.064.9 65.069.9 70.074.9 75.079.9 80.084.9 85.089.9 90.094.9 95.099.9 100.0104.9 105.0109.9 110.0114.9 115.0119.9 120.0124.9 125.0129.9 100.0104.9 105.0109.9 110.0114.9 115.0119.9 120.0124.9 125.0129.9 Size Category/mm b Ringhaddy Horse Mussels 16 14 Frequency/no units 12 10 8 6 4 2 0 50.054.9 55.059.9 60.064.9 65.069.9 70.074.9 75.079.9 80.084.9 85.089.9 90.094.9 95.099.9 Size Category/mm Figure 6.3.5 a) North Basin (N = 80) and, b)South Basin (N = 71) broodstock size frequency histograms. 6.3.4.2 Broodstock response to supplemental algal diet An experiment was carried out to assess if there were differences in the response to air exposure stimuli in conditioned and unconditioned mussels. An experiment was set up by placing 100 adult M. modiolus in running seawater. Half of the broodstock was given a supplement of Tetraselmis suecica using a peristaltic pump at a rate of 1.25 L/min while the other half had no supplemental feed. The tanks were drained after 3 days and the broodstock mussels left exposed to air overnight. The next morning water flow was resumed. Spawning was recorded 24 hours later in both holding tanks therefore there was no beneficial effect of adding algae to the holding tanks. 173 6.3.4.3 Description of spawning and the larval cycle During the first spawning trial North Basin broodstock mussels released an estimated total of total 8 million eggs, each female releasing an estimated 230,000 eggs. South Basin mussels released 13 million eggs, an estimated number of 546,000 eggs per female (Figure 6.3.6). Differences in numbers of spawned eggs between the North and South Basin broodstock populations were not statistically significant (ANOVA: F(1,4) = 3.453; p > 0.05) Once spawning had started it continued for almost a week. Spent gonads where observed in most animals after sacrificial Mean spawned eggs ± SD Millions sub-samples were taken. 8 7 6 5 4 3 North Basin (n=80) South Basin (n=70) 2 1 0 Figure 6.3.6 Oocyte count spawned from both broodstock M. modiolus used in the pilot hatchery. Fertilized egg diameter was 88.3 ± 2.35 μm while the first cleavage (two equal blastomeres and a polar body) was observed one hour after fertilization. Fertilization percentages were as high as 89% in some trials. Embryonic stages, similar to those recorded for M. modiolus by Schweinitz & Lutz (1976) and Flyachinskaya, & Naumov (2003), were documented (Figure 6.3.7). A 4cell stage consisting of one big macromere and three smaller micromeres was observed two hours after fertilization. The ciliated gastrula appeared in the 10 th hour and differentiated into a trocophore of approximately 70 μm in length. The first D174 shaped veligers appeared 24 to 48 hours after fertilization; mean length ± SD was 117 ± 19 μm. Early umbonated veligers were observed in the 15 th day; mean lengths ± SD ranged from 119 ± 17.76 μm to 129.6 ± 11.43 μm depending on the diet. Fully umbonated veligers appeared on the 18th day after fertilization, mean lengths varied from 130.2 ± 13.09 μm to 148.1 ± 17.91 μm. After 21 days veligers with a functional velum, reached maximum lengths of 220 μm and mean sizes of 179.8 ± 25.18 μm. Both incipient foot and eye spot were visible in very few individuals by the 30th day. Crawling eyed pediveligers with functional foot were observed for the first time 38 days after fertilization. The pediveligers were few and attained lengths of 350 to 400 μm. It was noticed that while some larvae seemed to be in an advanced stage of development, considerable numbers were still in the trocophore and ciliated D-larvae stages. Some researchers have also reported this phenomenon (Loosanoff & Davis 1963) suggesting that the larval development stage depends more on size than on age (Schweinitz & Lutz 1976). 175 A B D E G H C cl F I es sh v f Figure 6.3.7 Light micrographs of Modiolus modiolus larvae reared in Queen‟s University Marine Laboratory, Portaferry (2008-10): (A) t0: Fertilization; (B) t0 + 1h: First cleavage-trefoil stage (Da Costa et al. 2008): t0 + 2 h: blastomeres and polar lobe (pl) visible; (C)–(D) t0 + 10 h: 4–32-cell stages; (E) t0 + 16h: hatched blastula with long uniform cilia (cl); (F) t0 + 24 h: Trochophore; (G) 2 days: Prodissoconch I: D-shell veliger larva. Velum (v) and shell (sh) visible; (H) 15 days: Prodissoconch II: umbonated larva; (I) 38 days: Pediveliger larvae. Active foot (f) and eye spot (es) visible. Scale bars= 50 µm 6.3.4.4 Effect of diet in larval growth and survival The effect of different dietary regimes on growth and survival was tested during the trials in 2009. Mean shell length data (50 larvae measured on the 4 th, 8th, 10th, 13th, 15th and 17th day) for each feeding regime was subjected to a two-way ANOVA with replication showing no significant advantages of mixed diets over single-cell diets (F4, 10 = 2.05; p > 0.05). To measure the effect diet has on larval survival, all tanks were drained and larvae counted every second day during the 25 day period after fertilization. On the 25th day larvae fed with double or triple combination of algal species were most numerous: C.calcitrans + I. galbana T-ISO + T.suecica (40,000); 176 C.calcitrans and T. suecica (38000); and T-ISO and T.suecica (33000). Counts of larvae fed with single species diets were lower: C. calcitrans (19,000); I. galbana TISO (23000) (Figure 6.3.8). The relationship between larval survival and algal diet was tested using 2-way analysis of variance without replication. Mixed algal diets performance was not significantly better than single species diets (total larvae in each treatment 25 days from fertilization; F(4,71) = 2.28; p > 0.05). a) b) Figure 6.3.8 Influence of algal diet in a) growth and b) survival of M. modiolus larvae in hatchery trials. Treatments listed in Table 6.3.1. 177 6.3.4.5 Settlement The first settlement experiments were carried out in 2009. Monofilament net and scrubbing pads were placed in the larval tanks on the 25 th day to test whether the presence of settlement substrata would trigger metamorphosis. Swimming veligers were still present in the water column almost 2 months after the settlement experiment commenced. At this stage it was decided to check the experimental setups for M. modiolus spat. Each tank was drained through a 200 µm and 90 µm sieves. No settled spat was recorded in any of the sieves although empty veliger shells were retained in the 90 µm sieve. Each spat collector was carefully checked under the stereomicroscope but no spat was recorded in any of the six tanks used. In 2010 a repeated hatchery trial yielded the first crawling pediveligers by the 38 th day. M.modiolus shell and live clumps were used to replicate natural conditions. After 4 months left in an upwelling tank the shells were checked for spat. A total of four spat measuring 1.5 to 2 mm in length were collected leaving a settlement rate of 0.00026% [SR = (No. Settled spat/ No. Initial larvae) x100] (Beduschi et al. 2009). The presence of settled spat meant the full life cycle of M. modiolus was documented under laboratory conditions (Figure 6.3.9). The effect of microencapsulated MySpat® diets on spat growth could not be tested due to the low numbers of M. modiolus spat produced in the hatchery. 178 Figure 6.3.9 Life cycle of the horse mussel Modiolus modiolus as observed in the experimental hatchery set up by the MRRG (flow chart adapted from Helm et al. (2004) using original data and photographs from the present study). 6.3.5 Discussion 6.3.5.1 Hatchery cultivation of M. modiolus The pilot hatchery set up by the Modiolus Restoration Research Group was successful in describing the life cycle of M. modiolus in detail but very few larvae developed to pediveliger stage. Some aspects of the biology of M. modiolus in Strangford Lough need to be studied in more detail, particularly regarding its reproductive behaviour. Many temperate species require 6 to 8 weeks in the conditioning tanks before the spawning induction phase can commence (Helm et al. 2004). The fact that Strangford Lough M. modiolus breeding population seems to remain in a constant ripe stage throughout the year (Seed & Brown 1977; personal observations) made conditioning unnecessary. This fact, along with the constant presence of spat in the 179 population leads to the conclusion that M. modiolus might reproduce by releasing small amounts of gametes throughout the year without a clear seasonality as proposed by Seed & Brown (1977). This behaviour is very relevant to hatchery production of M. modiolus if we select broodstocks from populations in Strangford Lough. A clear knowledge of the natural phenomena that trigger spawning of M. modiolus in the wild is needed for the induction spawning process. A change in temperature usually prompts spawning in wild bivalve populations. The thermal cycling method usually triggers spawning in most bivalve species but the results obtained during our trials were not positive. Jasim (1976) successfully spawned M. modiolus using temperature shock but Schweinitz & Lutz (1976) and Flyachinskaya & Naumov (2003) also found broodstock M. modiolus unresponsive to changes in water temperature and other traditional spawning methods. Helm et al. (2003) state that if thermal cycling does not trigger spawning, the gametes are probably not fully mature. The only way we consistently triggered spawning in the broodstock was using stress by exposure to air. The method is not a replication of a natural phenomena as the broodstock from Strangford Lough is fully subtidal and it never gets exposed to air. However the phenomenon has been observed elsewhere (C.A. Richardson pers. comm.). This may explain why metamorphosis was recorded only once and why the rate of individual larval development was highly variable with several stages present in the culture containers until the culture collapsed. If spawning was the consequence of a highly distressing event it is possible that the condition of the gametes was not ideal for hatchery experiments. When pediveligers were obtained the full cycle lasted for more than 1 month which matches observations of a long planktonic stage for M. modiolus by Ockleman (1965) and Flyanchinskaya & Naumov (2003). Schweinitz & Lutz (1976) recorded pediveligers after 19 days but the sea water where the larvae were kept was heated to 21°C. In the present study it was decided to use ambient temperature and document the larval cycle as it is likely to happen under natural conditions in Strangford Lough. This information will be very useful in a larval dispersal model specific for Strangford Lough. The provision of suitable settlement substrata for M. modiolus hatchery production is another problematic area. Field studies (Section 6.2) suggested that M. modiolus spat rarely settle outside the matrix created by adult live mussels. Substratum preference experiments in the wild recorded significant differences in recruitment 180 levels between inert substrata (empty shells, synthetic fibres) and clumps of live mussels (Section 6.2). These results were also observed during the hatchery trials with no settlement in any of the spat collectors introduced in the larval tanks. 6.3.5.2 Economics of seed mussel production Hatchery production of commercial mussel species is considered uneconomic due to the low economic return per unit produced; therefore most mussel industries are based on natural spatfall (Lucas 2003). The low survival rates of hatchery reared bivalves once transplanted is also an important factor considering the high production costs involved (Caddy & Defeo 2003; Maguire et al. 2007). The running costs of a pilot M. modiolus hatchery are as high if not higher than commercial mussel hatchery considering the lack of previous knowledge regarding basic aspects of the biology of the species. Setting up and running costs of the M. modiolus experimental hatchery were in excess of £6,500 per month, including: Production of microalgae Running costs of sea water system Electricity Equipment Boat usage to collect fresh broodstock Staff salaries (technical, boatman, divers) General hatchery costs in the Republic of Ireland at €11,500 per month in 2007 (Maguire et al. 2007) are comparatively well above the costs in which the pilot M. modiolus hatchery incurred during its trials in 2008-10. Economic benefits of a hatchery solely focused in producing spat for ecological restoration are harder to estimate if compared to a commercial hatchery where the returns are based in number of spat (in their millions) sold to grow at commercial rates for on-growing for human consumption. The costing shown is exclusive of intermediate cultivation costs as not enough seed was produced to merit setting up a M. modiolus spat nursery. If that had been possible the costs would have been much higher considering the slow growth of the 181 species as it would probably take one or two years to produce spat of just 10 mm as ageing data collected during the project has confirmed. If relaying hatchery produced spat was considered to be an option spat should be at least 40 mm, considering the size at which most mussels escape predation (Roberts 1975). 6.3.6 Conclusions The production of M. modiolus spat in a dedicated hatchery has nevertheless some benefits over the other restoration approaches, namely cultch deployment and translocation. It is almost non-disruptive and the spat produced comes from the local population. Collection of natural spat using collectors deployed in different locations in Strangford Lough was very poor due to the specific settlement preferences of M. modiolus (Section 6.2); therefore population enhancement by collecting natural spat is not a viable option. Translocation of locally sourced M. modiolus disrupts the few „good‟ beds remaining while sourcing the mussels from outside the Lough would require genetic studies and strict controls to avoid the introduction of non-native species such as Crepidula fornicata and toxic dinoflagelates. The nominal production of spat by the experimental hatchery was nowhere near the millions that are needed to attempt an experimental reseeding programme as survival of relaid spat in the field is usually very low. The high costs associated with running the hatchery operations compared to the poor return in seed means hatchery production of M. modiolus is not a viable restoration option at this stage. 182 7. Projection for recovery of ‘Favourable Conservation status’ (Undertaking 8) 7.1 Summary Most of the assessment criteria suggest that M. modiolus biogenic reefs, particularly biotope SS.SBR.SMus.ModCvar in Strangford Lough remain in unfavourable conservation status. However, at some sites, notably Round Island Pinnacle, Colin Rock, north to Craigyouran „good‟ condition exemplars of biotope SS.SBR.SMus.ModHAs remain. Because results from short-term temporal monitoring showed no clear trends, it is not possible to develop a model based on the current study to predict when favourable conservation status of M. modiolus biotopes will be restored in Strangford Lough. Published studies from New Zealand and Canada suggest that impacted bivalve biogenic reefs may take extended periods to recover after the cessation of fishing activities. Factors affecting successional regeneration of bivalve biogenic reefs include the period of non-disturbance, proximity of propagule sources and hydrodynamic influences on propagule dispersal. In Strangford Lough, much of the degraded M. modiolus habitat lies within 10-15 km of sources of propagules from the remaining beds; based on the recovery of oyster and mussel reefs in New Zealand this suggests that signs of natural recovery might be expected within 20 years in Strangford Lough, provided there is no further disturbance. 183 7.2 Introduction The long-term objective of the Modiolus Restoration Plan (DOE/DARD) (Anon 2005) is: “to restore the Strangford Lough Modiolus biogenic reef feature to „Favourable Conservation status‟.” Undertaking 8 of the current project is to develop projections, based on temporal monitoring, for when „Favourable Conservation status‟ might be achieved. 7.3 Current conservation status Table 7.1 measures current status of main M. modiolus biotopes in Strangford Lough against the habitats directive definitions of „Favourable Conservation status‟ of both the habitat (the biotope) and species. Most of the assessment criteria suggest that the majority of M. modiolus biogenic reefs, particularly biotope SS.SBR.SMus.ModCvar in Strangford Lough remain in unfavourable conservation status. At some sites the biotope SS.SBR.SMus.ModHAs shows increasing fragmentation. „Good‟ condition sites of this biotope at e.g. Craigyouran and West of Round Island Pinnacle are not in pristine condition when compared with other beds in the U.K. However, these probably represent the best remaining M. modiolus communities in the Lough. 184 Table 7.1 Assessment of the current conservation status of M. modiolus biotopes in Strangford Lough (December 2010) Definitions of ‘Favourable Conservation Status’ Biotopes Natural Habitat Its natural range is stable or increasing. SS.SBR.SMus.ModCvar Continuing to decline in distribution and condition. The specific structure and functions necessary for its long-term maintenance exist and are likely to continue for the foreseeable future. The conservation status of its typical species is favourable. Because there has been continued contraction in the range of its „foundation‟ species, M modiolus, this condition is not met. Species (Modiolus modiolus) Population dynamics indicate it is maintaining itself on a long-term basis as part of its natural habitat Its natural range is not declining or likely to decline in the foreseeable future There is a sufficiently large habitat to maintain it on a long-term basis SS.SBR.SMus.ModHAs/ModT At some sites this biotope shows increasing fragmentation. „Good‟ condition sites at e.g. Craigyouran and West of Round Island Pinnacle are not in pristine condition when compared with other beds in the U.K. However, these probably represent the best remaining M. modiolus communities in the Lough. This condition is only met at a small number of sites. Although many species recorded in previous surveys are still present and presumably selfmaintaining, species diversity has declined between 2003 and 2010. In addition, key species for this biotope (Chlamys varia and Aequipecten opercularis) are missing or underrepresented. Most species recorded in previous surveys are still present and presumably self-maintaining. Species diversity has not declined between 2003 and 2010. Natural recruitment is very poor. Natural recruitment is high. Most of the recent (since 2003) contraction in range has been in the northern basin. Range contraction (since 2003) less evident than in the northern basin. Based mainly on substratum characteristics, MAXENT modelling suggests that suitable habitat exists over much of its historical range. Based mainly on substratum characteristics, MAXENT modelling suggests that suitable habitat exists over much of its historical range. 185 7.4 Projection for achieving „Favourable Conservation Status‟. Because results from short-term temporal monitoring showed no clear trends, it is not possible to develop a model based on the current study to predict when favourable conservation status of M. modiolus biotopes will be restored in Strangford Lough. However, there is relevant ongoing research led by AFBI into the development of dynamic ecosystem carrying capacity models for the five sea loughs in the north of Ireland. This research culminated in the publication of Sustainable Mariculture in northern Irish Lough Ecosystems (SMILE) (Ferreira et al., 2007).Key objectives of SMILE were to: „establish functional models at the lough scale, describing key environmental variables and processes, aquaculture activities and their interactions evaluate the sustainable carrying capacity for aquaculture in the different loughs, considering interactions between cultivated species, targeting marketable cohorts, and fully integrating cultivation practices examine the effects of overexploitation on key ecological variables examine bay-scale environmental effects of different culture strategies.‟ During the course of the current project, the SMILE model for Strangford Lough was expanded to incorporate additional species, Modiolus modiolus, Ostrea edulis and Pecten maximus. This will allow carrying capacity limitations for these species under different hypothetical scenarios to be determined (Service et al., in prep.). Preliminary results indicate that in Strangford Lough, maximal feeding rates in M. modiolus (weight-corrected) are significantly lower than in the blue mussel, Mytilus edulis, which is consistent with the slower growth rate of the former. A scenario was run, where historical M. modiolus areas were populated at a “pristine” density of 50 individuals m-2. The model predicts that M. modiolus in areas close to the mouth of the lough will grow more quickly; this may be due to enhanced habitat suitability although it will be compounded by density dependent effects. The model also suggests that food availability is unlikely to be a factor limiting its recovery. (Service et al. in prep). In addition, two published studies provide invaluable insight intochanges in benthic communities, which follow the cessation of fishing in areas historically impacted by mobile fishing gear in New Zealand (Cranfield et al. 2004) 186 and in Canada (Kenchington et al. 2007). These studies are highly relevant to the present study because impacted communities in both cases included species of horse mussel, M. modiolus in Canada and M. areoltatus in New Zealand that had been damaged by scallop and oyster dredging respectively. Kenchington et al. (2007) documented significant changes in the benthic community over a 30-year period and suggested that these were primarily due to the physical impacts of mobile fishing gear. However, they did not document habitat recovery. By contrast, Cranfield et al. (2004) provide a model of biogenic habitat regeneration from bare substrate. Succession from was described in 5 stages: 1) settlement of initial colonising epifauna and infauna; 2) settlement of Modiolus areolatus and Ostrea chilensis; 3) mussel and oyster density increase; 4) diversity and biodeposition increase; 5) regeneration of the climax biogenic reef. Factors affecting this successional regeneration include the period of non-disturbance, proximity of propagule sources (Table 7.2; Cranfield et al. 2004) and hydrodynamic influences on propagule dispersal (Ayata et al. 2009). Sedimentation and sediment mobility have also been identified as potentially important factors which may influence the survival and recovery of M. modiolus biogenic reefs (Service 1990; Strong and Service 2008) and should be investigated further. Table 7.2 Relationships between factors affecting habitat regeneration and habitat complexity (Modified from Cranfield et al. 2004) Habitat Complexity Rank Height and form of habitat Substratum characteristics Estimated time since fished (years) Estimated nearest source of propagules 1 2 Sediment Sediment 0 50 >5km ~20km 3 Mud and sand Pebble gravel, fine sand Pebble gravel, sand Fine sand ~20cm biogenic reefs ~20cm biogenic reefs Biogenic reefs Fine sand 30-40cm high, 3-20m long 20 10-15km 20 <1km 12 <1km 4 5 187 In Strangford Lough, much of the degraded M. modiolus habitat lies within 10-15 km of sources of propagules from the remaining beds; however, a timescale for natural recovery in Strangford Lough can not be predicted unless there is no further disturbance. Historically there was a small fishery for Ostrea edulis in Strangford Lough, which collapsed around the 1900s (Smyth et al. 2009). Recovery of O. edulis in the low intertidal in Strangford Lough since 1998 was probably attributable to the presence of high density commercial stocks in the 1990s, again demonstrating the importance of propagules in community/species regeneration (Kennedy & Roberts 2006). No sub-littoral oysters were recorded in the Lough during SLECI in 2003. However, O. edulis was recorded at a number of sublittoral sites during the current project (personal observations, MRRG); in addition, Chlamys varia was observed in empty oyster shells at these sites. This observation may be comparable to stage 2 succession described by Cranfield et al. (2004). While Table 7.1 may appear to paint a bleak picture for the potential recovery of the M. modiolus biogenic reef feature in Strangford Lough, the experience in New Zealand suggests that biogenic reefs comprising Ostrea sp. and Modiolus sp. may recover over long periods if undisturbed. In addition, a number of positive elements have emerged from the current project. First, species richness remains high and some sites in both the north and south basins show evidence of natural recruitment. Second, intervention involving translocation of M. modiolus onto cultch shows a great potential to kick start the regeneration process. These positive elements of the study form the basis of the recommendations below. 188 8 Recommendations (Undertaking 3) 8.1 Options Do nothing Continue to deliver the DARD/DOE Modiolus Restoration plan. 8.2 Recommendations Recommendations below would enable DARD/DOE to continue to deliver the Modiolus Restoration plan and follow its three essential elements. 8.2.1 PROTECTION Maintain the ban on the use of mobile fishing gear MRRG recommend that a total protection zone is established below the 10m contour line between: Castle Island to Gransha Point in the North and the Southern tip of Island Taggart to Kate‟s Pladdy in the South (Fig 8.1). Rationale: The recommendation meets the first objective of the the Modiolus Restoration Plan agreed by DOE and DARD: „to identify, map and introduce total protection for the remaining Modiolus biogenic reef sites within i year of the adoption of this plan; damaged biogenic reefs will also be identified and protected from further damage‟. The proposed total protection zone: Contains bulk of remaining M. modiolus communities Contains experimental restoration cultch site 189 Includes a significant proportion of habitat suitable for M. modiolus biotopes (ModCvar and ModHAs/ModT) based on habitat suitability models. Issues: Impacts on current stakeholder activities This issue is without the scope of this report and fall within the responsibilities of the competent authorities, DOE and DARD. 8.2.2 INTERVENTION Establish at least one new artificial reef within the proposed non-disturbance zone using weathered cultch. The reef site should be selected on the basis of habitat suitability and larval dispersal modelling, be located within both the proposed non-disturbance zone and the historical distribution of the Modiolus modiolus beds with Chlamys varia, sponges, hydroids and bryozoans (SS.SBR.SMus.ModCvar) biotope. Ideally large numbers of adult mussels should be translocated onto cultch in the area(s) selected for translocation. Based on preliminary results from the current study, which found no significant difference in survival of mussels translocated on to elevated or flattened cultch or onto unmodified substrate the use of cultch may be redundant. However, sourcing and deployment of cultch should be budgeted into any further intervention efforts. In addition, because sourcing mussels for translocation remains a problem (see below) the best approach might be to concentrate existing mussels into larger patches. This would stabilse mussel patches due to the clumping behaviour of mussels and overcome Allee effects whereby reproductive success decreases with population density. Rationale: Experimental trials in current project show that: • Artificial reefs stabilise quickly • Translocated mussels show high survival 190 • Reefs are rapidly colonised by epifauna Issues: • The key issue involving translocation of mussels is that of acquiring sufficient quantities of mussels to increase the chances of success. This would necessitate collecting mussels from outside Strangford Lough for this purpose. This was considered when the pilot reef experiment was initiated during the present project. The proposal was rejected on the grounds that 1) M. modiolus beds in the western Irish sea (the nearest source stocks) are themselves already under threat (Goodwin et al. 2011); 2) there would be a risk of introducing pathogens and alien species; 3) introduced mussels might not be genetically compatible with populations in Strangford Lough (see for example Maggs 2008). However, because this project has successfully translocated mussels in small-scale trials, the potential risks and benefits of such intervention should be re-evaluated with a view to undertaking translocation on a large scale. • 8.2.3 Cost MONITORING Establish an annual programme to monitor: • status of natural biogenic reefs • recruitment & succession on established experimental reef • recruitment & succession on proposed experimental reef • selected historical sites where M. modiolus no longer occurs Rationale: Longer time frame is required to demonstrate: positive or negative changes in natural reefs (natural recovery) • Effectiveness of artificial reefs Methodology • Status of natural biogenic reefs: 191 Transect surveys should be repeated every 6 months at the same locations studied during the SLECI and MRRG projects using the same methodology. The surveying method will involve deploying a 100 m leaded line to mark the transect position at each survey areas. Divers will move along the transects taking representative still digital photographs of the seabed at regular 5 m intervals. M. modiolus presence will also be recorded at each 5 m tag. The digital photographs will be analyzed in the laboratory documenting the epifaunal assemblage, determining the abundance and percent cover of each taxon observed. M. modiolus density and associated infaunal community will be studied by means of an annual total removal sampling programme. • Recruitment & succession on established and proposed experimental reefs: A photographic and video transect survey should be carried out every 6 months to document faunal community succession. Photographs should be analysed for M .modiolus densities and mortalities while associated epifauna should be identified, counted and percentage coverage calculated. A video survey should also be carried out to document condition and structure of the experimental reef and to record the presence of species missed during the photographic survey, particularly fish and other mobile fauna. An annual total removal survey using smaller 0.0625 m2 to minimize disturbance should also be carried out. Issues: • Costs 192 Figure 8.1 Distribution of Modiolus modiolus in Strangford Lough in 2010 based on surveys conducted by the Modiolus Restoration Research Group (Inset). M. modiolus reefs were recorded at 123 of 442 sites surveyed and is the basis of the recommendation to establish a total protection zone below the 10m depth contour, between Castle Island to Gransha Point in the North and the Southern tip of Island Taggart to Kate‟s Pladdy in the South (Main figure). The recommendation aims to meet the first objective of the Modiolus Restoration Plan agreed by DOE and DARD: „to identify, map and introduce total protection for the remaining Modiolus biogenic reef sites within 1 year of the adoption of this plan‟. The map also indicates: The position of „good‟ condition sites, at Craigyouran and Round Island Pinnacle The experimental cultch site Strangford Lough (Sea Fisheries Exclusion Zones) Regulations (Northern Ireland) 2011 No. 36, Introduced 14th March 2011 are also illustrated. 193 STRANGFORD LOUGH ARDS PENINSULA IRISH SEA Proposed M. modiolus protected area Sea Fisheries Exclusion Zones March 2011 Experimental M. modiolus reef site MRRG survey records (2008-2010) M. modiolus present M. modiolus absent 194 9.0 References 92/43/EEC Council Directive of 21 May 1992 on the conservation of natural habitats and wild flora and fauna, AFBI (2011). Data available from: <http://www.afbini.gov.uk/index/services/servicesspecialist-advice/coastal-science/coastal-monitoring/monitored-sites/nstrangford.htm> Anderson, M.J, Gorley, R.N., and Clarke K.R. (2008) PERMANOVA+ for Primer: Guide to software and statistical methods, 1st edition. PRIMER-E, Plymouth, UK Anderson, R. P., Lew, D. & Peterson, A. T. (2003) Evaluating predictive models of species‟ distributions: criteria for selecting optimal models. Ecological Modelling 162: 211–232. Anon (2005) Strangford Lough Modiolus Biogenic Reef DOE/DARD Restoration Plan. DOE/DARD, Northern Ireland. 18pp. Anwar, N.A., Richardson, C.A. & Seed, R. (1990) Age determination, growth rate and population structure of the horse mussel Modiolus modiolus. Journal of the Marine Biological Association UK, 70: 441-457. Ayata S-D., Ellien C., Dumas F., Dubois S. and Thiébaut E. (2009) Modelling larval dispersal and settlement of the reef-building polychaete Sabellaria alveolata: Role of hydroclimatic processes on the sustainability of biogenic reefs. Continental Shelf Research 29: 1605 -1623. Bartol, I.K. and Mann, R. (1997) Small-scale settlement patterns of the oyster Crassostrea virginica on a constructed intertidal reef. Bulletin of Marine Science 61 (3): 881-897 Bayne, B.L. (1965) Growth and the delay of metamorphosis of the larvae of Mytilus edulis (L.). Ophelia, 2(1), 1-47. Bayne, B.L.(1976) The biology of mussel larvae. In: Marine Mussels: their Ecology and Physiology (ed. B.L. Bayne), pp. 13-65. Cambridge University Press, Cambridge. Beck, M.W., Brumbaugh, R.D., Airoldi, L., Carranza, A., Coen, L.D., Crawford, C., Defeo, O., Edgar, G.J., Hancock, B., Kay, M., Lenihan, H., Luckenbach, M.W., Toropova, C.L., Zhang, G. (2009) Shellfish Reefs at Risk: A Global Analysis of Problems and Solutions. The Nature Conservancy, Arlington VA. 52 pp. 195 Beduschi, P., de Melo, C.M.R. & Ferreira, J.M. (2009) The influence of techniques of larvae rearing and seed collectors on the survival rate and recovery efficiency of the Brown Mussel Perna perna (L.) in laboratory. Brazilian Archives of Biology and Technology: 52(1), 145-152. Boyd, R.J. (1973) The relation of the plankton to the physical, chemical and biological features of Strangford Lough, Co. Down. Proceedings of the Royal Irish Academy 73B: 317-353 Bradshaw C., Veale L. O., and Brand A. R. (2002) The role of scallop-dredge disturbance in long-term changes in Irish Sea benthic communities: a re-analysis of an historical dataset. Journal of Sea Research 47:161-184 Brown, R.A. (1984) Geographical variations in the reproduction of the horse mussel, Modiolus modiolus (Mollusca:Bivalvia). Journal of the Marine Biological Association of the United Kingdom 64: 751-770. Brown, R. A. (1989) Bottom trawling in Strangford Lough: Problems and Policies. in ten Halers, C. C., and Bijlsma, A. editor, Proceedings from the 3rd North Sea Seminar. Rotterdam, Netherlands. Brown, R. A. & Seed, R. (1977). Modiolus modiolus (L.) - An autoecological study. Pages 93-100 in Keegan, B. F., O'Ceidih, P.O. & Boaden, P.J.S., editor. Biology of Benthic Organisms. Proceedings of the 11th European Symposium on Marine Biology, Pergamon Press, Oxford. Galway, Ireland. Brown, R.A., Seed, R. and O‟Connor, R.J. (1976) Comparison of relative growth in Cerastoderma (=Cardium) edule, Modiolus modiolus, and Mytilus edulis (Mollusca-Bivalvia). Journal of Zoology 179: 297-315. Brumbaugh, R.D., Beck, M.W., Coen, L.D., Craig, L. and Hicks, P. (2006) A practitioner’s guide to the design and monitoring of shellfish restoration projects: an ecosystems services approach. The Nature Conservancy, Arlington, VA. Bushek, D., Richardson, D. Bobo, M.Y. and Coen, L.D. (2004) Quarantine of oyster shell cultch reduces the abundance of Perkensus marinus. Journal of Shellfish Research 23: 369-373. Caddy, J.F. and Defeo, O. (2003) Enhancing or restoring the productivity of natural populations of shellfish and other marine invertebrate resources. FAO Fisheries Technical Paper 448. Food and Agriculture Organization of the United Nations. 159pp. 196 Clarke, K.R., Warwick, R.M. (2001) Change in marine communities: an approach to statistical analysis and interpretation, 2nd edition. PRIMER-E, Plymouth, UK Cork, M., Adnitt, C., Staniland, R. and Davison, A. (2006) Creation and Management of Marine Protected Areas in Northern Ireland. Environment and Heritage Service Research and Development Series. No. 06/18. Available from: http://www.doeni.gov.uk/niea/mpa-report_amended.pdf Coen, L.D., Brumbaugh, R.D., Bushek, D., Grizzle, R., Luckenbach, M.W., Posey, M.H., Powers, S.P. & Tolley, S.G. (2007) Ecosystem services related to oyster restoration. Marine Ecology-Progress Series 341: 303-307. Cole, V.J. (2010) Alteration of the configuration of bioengineers affects associated taxa. Marine Ecology-Progress series 416: 127-136. Comely, C.A. (1978) Modiolus modiolus (L.) from the Scottish west coast, Ophelia 17: 167-193 Comely, C.A. (1981) The physical and biochemical condition of Modiolus modiolus (L.) in selected Shetland voes. Proceedings of the Royal Society of Edinburgh 80B: 299-321. Connor, D.W, Allen, J.H., Golding, N., Howell, K.L., Lieberknecht, L.M., Northen, K.O & Becker, J.B. (2004) The Marine Habitat Classification for Britain and Ireland Version 04.05. JNCC, http://www.jncc.gov.uk/MarineHabitatClassification Council of the European Communities (1992). Council Directive 92/43/EEC on the conservation of natural habitats of wild fauna and flora. The Council of the European Communities, Brussels. Cranfield H.J., Rowden, A.A., Smith D.J., Gordon, D.P. and Michael K.P. (2004) Macrofaunal assemblages of benthic habitat of different complexity and a `proposition of a model of biogenic reef habitat regeneration in Foveaux Strait, New Zealand. Journal of Sea Research 52: 109-125. Cranfield, H.J., Carbines, G., Michael K.P., Dunn, A., Stotter, D.A. & Smith, D.J. (2001). Promising signs of regeneration of blue cod and oyster habitat changed by dredging in Foveaux Strait, southern New Zealand. New Zealand Journal of Marine and Freshwater Research 35: 897-908. Da Costa, F., Darriba, S. and Martínez-Patiño, D. (2008) Embryonic and larval development of Ensis arcuatus (Jeffreys, 1865) (Bivalvia Pharidae). Journal of Molluscan Studies 74 (2): 103-109. 197 Davenport, J. and Kjørsvik, E. (1982) Observations on a Norwegian intertidal population of the horse mussel Modiolus modiolus. Journal of Molluscan Studies 48: 370-371. Environment and Heritage Service (2001) Strangford Lough SAC/SPA Management Scheme. Available from: http://www.doeni.gov.uk/niea/strangfordmanagementscheme.pdf Erwin, D. G. (1970) A diving survey of Strangford Lough: the benthic communities and their relation to substrate-a preliminary account. Pages 215-224 in B. F. Keegan, O'Ceidih, P.O. and Boaden, P.J.S., editor. Biology of Benthic Organisms. Pergamon Press, Oxford. Erwin, D. G. (1986) Strangford Lough benthos and the marine community concept. Unpublished PhD Thesis. Queen's University, Belfast. 172pp. Erwin, D.G., Picton, B.E., Connor, D.W., Howson, C.M., Gilleece, P. and Bogues, M.J. (1986). The Northern Ireland Sublittoral Survey. Ulster Museum Belfast: HMSO. Erwin, D.G., Picton, B.E., Connor, D.W., Howson, C.M., Gilleece, P. and Bogues, M.J. (1990) Inshore marine life of Northern Ireland. Belfast, HMSO for Department of the Environment (Northern Ireland). Ferreira, J.G. and fourteen other authors (2007) SMILE, Sustainable Mariculture in northern Irish Lough Ecosystems. Ed. IMAR Institute of Marine Research, 100pp. Flyachinskaya, LP.. and Naumov, A.D. (2003). Distribution and larval development in the horse mussel Modiolus modiolus (Linnaeus, 1758) (Bivalvia, Mytilidae) from the White Sea. Proceedings of the Zoological Institute of the Russian Acadademy of Science 299: 39-50. Galley, T.H., Batista, F.M., Braithwaite, R., King, J. and Beaumont, A.R. (2010) Optimisation of larval culture of Mytilus edulis (L.). Aquaculture International 18: 315-325. Goodwin, C., Picton, B., Breen, J., Edwards H. And Nunn, J. (2011) Sublittoral Survey Northern Ireland (2006-2008): A review of the status of Northern Ireland priority species of marine invertebrates. Northern Ireland Environment Agency Research and Develpment Series No11/01. 152pp. Gibson, L.A., Wilson, B.A., Cahill, D.A., Hill, J., (2004). Spatial prediction of rufous bristlebird habitat in a coastal heathland: a GIS-based approach. Journal of Applied Ecology 41: 213-223. 198 Gosling, E. (2003) Bivalve Molluscs: Biology, Ecology and Culture. Fishing New Books, Oxford. Griffith, B., Scott, J.M. Carpenter, J.W. & Reed C., 1989. Translocation as a species conservation tool: status and strategy. Science 245: 477-480. Guisan, A., Zimmerman, N.E. (2000) Predictive habitat distribution models in ecology. Ecological Modelling 135:147-186. Hastie, L.C, Tarr, E.C., al-Mousawi, B. and Young, M.R. (2010) Medium-term recruitment patterns in Scottish freshwater pearl mussel Margaritifera margaritifera populations. Endangered Species Research 11: 21-33 Helm, M.M., Bourne, N. and Lovatelli, A. (2004) Hatchery culture of bivalves - A practical manual. FAO Publishing. Rome, 177 pp. Héral, M., (1990). Traditional oyster culture in France. Pp. 342-387 in G. Barnabe (ed.), Aquaculture, Volume 1. Ellis Horwood, New York. Hochburg, M.E. & Ives, A.R. (1999) Can natural enemies enforce geographical range limits? Ecogeography 22: 268-276. Holt, T. J., Rees, E.I., Hawkins, S.J. and Seed, R. (1998) Biogenic Reefs (volume IX). An overview of dynamic and sensitivity characteristics for conservation management of Marine SACs. Scottish Association for Marine Science (UK Marine SAC Project), Liverpool. Howson, C.M. & Picton, B.E. (eds) (2000). The species directory of the marine fauna and flora of the British Isles and surrounding seas. Ulster Museum and The Marine Conservation Society, Belfast and Ross-on-Wye. CD-ROM Edition http://europa.eu.int/comm/envitonment/nature/habdir.htm Inve Ltd. (2009) Available from: http://www.inve.com/INVEAquaculture/English/Products/Shellfishhatcheries/Dry+diets/MySpat/page.aspx/1151 Jasim, A.K. (1986) Some ecological aspects of Modiolus modiolus populations off the south-east of the Isle of Man. Unpublished PhD Thesis. University of Liverpool. Jasim, A.K. and Brand, A.R. (1989) Observations on the reproduction of Modiolus modiolus in Isle of Man waters. Journal of the Marine Biological Association UK 69:373-385. 199 JNCC (2011) MNCR SACFOR abundance scales. Available from: http://jncc.defra.gov.uk/ProtectedSites/SACselection/n2kforms/UK0016618.pdfUK SAC data form Jones N S (1952) The bottom fauna off the south of the Isle of Man. Journal of Animal Ecology 20: 132-144. Jørgensen, C.B. (1946) Lamellibranchia. Reproduction and larval development of Danish bottom invertebrates. Medd. Komm. Dan. Fisk. Havundersøg., Copenhagen, Series-Plankton 4: 277-311. Kenchington, E. L., Kenchington, T. J., Henry, L., Fuller, S., Gonzalez, P. (2007) Multi-decadal changes in the megabenthos of the Bay of Fundy: the effects of fishing. Journal of Sea Research 58: 220-240. Kennedy, R.J., Roberts, D. (2006) Commercial oyster stocks as a potential source of larvae in the regeneration of Ostrea edulis in Strangford Lough, Northern Ireland. Journal of the Marine Biological Association of the United Kingdom 86 (1): 153159. Koivisto M E and Westerbom M. (2010) Habitat structure and complexity as determinants of biodiversity in blue mussel beds on sublittoral rocky shores. Marine Biology 157: 1463–1474 Kraeuter, J.N., Kennish, M.J., Dobarro, J., Fegley, S.R., Flimlin Jr., G.E. (2003) Rehabilitation of the Northen quahog (Hard Clam) (Mercenaria mercenaria) habitats by shelling-11 years in Barnegat Bay, New Jersey, Journal of Shellfish Research 22(1): 61-67. Laing, I. (1987) The use of artificial diets in rearing bivalve spat. Aquaculture 65(3-4); 243-249. Leard, R.L., Dugas, R. & Berrigan M. (1999). Resource Management Programs for the Eastern Oyster, Crassostrea virginica, in the U.S. Gulf of Mexico, Past, Present, Future In : Luckenbach, M.W., Mann, R., Wesson, J.A. (Eds.), Oyster Reef Habitat Restoration: A Synopsis and Synthesis of Approaches. Virginia Institute of Marine Science Press, Gloucester Point, VA, pp. 159–170. Lilleskare, J. (1905). Om Forekomst av Oskajael paa Dybvand. Norks Fiskeritidende, 98-109 Liu, A.Y., Schisterman, E.F. & Wu, C.Q. (2005) Nonparametric estimation and hypothesis testing on the partial area under receiver operating characteristic curves. Communications in statistics- theory and methods 34 (9-10): 2077-2088. 200 Loosanof, V.L. and Davis, H.C. (1963) Rearing of bivalve molluscs. Advances in Marine Biology 1: 1-136 Lucas, J. (2003) Bivalves. In: Aquaculture. Farming aquatic animals and plants (Ed. by J.S. Lucas and P.C. Southgate), pp. 157-76. Blackwell Publishing Ltd., Oxford Maggs, C. (2008) Special Protection and Local Action for Species and Habitats (SPLASH) 2004-2008. Final Report. Queen‟s University Belfast. Available from: www.marlin.ac.uk/pdf/SPLASH%20Final%20Report.pdf Magorrian, B. H., and M. Service. (1998) Analysis of underwater visual data to identify the impact of physical disturbance on horse mussel (Modiolus modiolus) beds. Marine Pollution Bulletin 36: 354-359. Magorrian, B.H., Service, M. and Clarke, W. (1995) An acoustic bottom classification survey of Strangford Lough, Northern Ireland. Journal of the Marine Biological Association of the United Kingdom 75: 987-992. Maguire, J.A., Knights, T., Burnell, G., Crowe, T.C., O‟Beirn, F., McGrath, D., Ferns, N., McDonough, N., McQuaid, N., O‟Connor, B., Doyle, R., Newell, R., Seed, R., Smaal, A., O‟Carroll, T., Walson, L., Dennis, J. and O‟Cinneide, M. (2007). Management recommendations for the sustainable exploitation of mussel seed in the Irish Sea. Marine Environment and Health Series, 31. Mair, J. M., Moore, C. G., Kingston, P. F., Harries, D. B. (2000) A review of the status, ecology and conservation of horse mussel Modiolus modiolus beds in Scotland. Scottish Natural Heritage Commissioned Report F99PA08. Mann, R. and Evans, D. (2004) Site selection for oyster habitat rehabilitation in the Virginia portion of the Chesapeake Bay. Journal of Shellfish Research 23(1): 4149. Meadows, P.S. and Shand, P. (1989) Experimental analysis of byssus thread production by Mytilus edulis and Modiolus modiolus in sediments. Marine Biology 101: 219-226. Mitchell, A. and Service, M. (2003). Northern Ireland Nearshore Habitat Mapping Project. Modiolus Restoration Research Group (2008). Unpublished reports. Moore, C. G., Saunders, G. R., Harries, D. B., Mair, J. M., Bates, C. R., Lyndon, A. R. (2006) The establishment of site condition monitoring of the subtidal reefs of Loch Creran SAC. Scottish Natural Heritage Commissioned Report No. 151 201 Nunn, J. (1994) The Marine Mollusca of Ireland 1. Strangford Lough, Co. Down. Irish biogeographical society. Bulletin No. 17(2): 23-214. Ocklemann, K.W. (1965) Developmental types in marine bivalves and their distribution along the Atlantic coast of Europe. In: Proceedings of the first European Malacological Congress, London 1962 25-35. Olsson, O. & Rogers, D.J. (2009) Predicting the distribution of a suitable habitat for the white stork in Southern Sweden: identifying priority areas for reintroduction and habitat restoration. Animal Conservation12: 62-70. Palumbi SR, Sandifer PA, Allan JD, Beck MW, Fatin DG, Fogarty MJ, Halpern BS, Incze LS, Leong JA, Norse E, Stachowicz JJ, Wall DH (2009) Managing for ocean biodiversity to sustain marine ecosystem services. Frontiers in Ecology and the Environment 7(4): 204-211. Peterson, C.H., Summerson, H.C. and Leuttich Jr.,R.A. (1996) Response of bay scallops to spawner transplants: a test of recruitment limitation. Marine Ecology Progress Series 264: 249-264. Phillips, S.J., Anderson, R.P., Schapire, R.E. (2006) Maximum entropy modelling of species geographic distributions. Ecological Modelling 190; 231-259. Phillips, S.J., Dudlík, M. (2008) Modelling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31: 161-175. Plass-Johnson, J.G., McQuaid, C.D. & Porri, F. (2010) Top-down effects on intertidal mussel populations: assessing two predator guilds in a South African marine protected area. Marine Ecology-Progress Series 411: 149-159. Powers, S., Peterson, C., Grabowski, J. & Lenihan, H. (2009) Success of constructed oyster reefs in no-harvest sanctuaries: implications for restoration. Marine Ecology Progress Series 389: 159-170 Rees, C.B. (1950). The identification and classification of lamellibranch larvae. Hull Bulletin of Marine Ecolology 4 (27):21-46. Rees, E. I. S. (2005). Assessment of the status of horse mussel (Modiolus modiolus) beds in the Irish Sea off NW Anglesey. Llanfairpwll. Rees, I. (2009) An assessment of M. modiolus beds in the OSPAR area. JNCC Report draft. 202 Rees, I. (2009) Background document for Modiolus modiolus beds. Report prepared on behalf of the Joint Nature Conservation Committee (JNCC) for the OSPAR COMMISSION [OSPAR Publication Number 425/2009] 28pp. Rees, I., Sanderson, W.G., Mackie A.S.Y and Holt, R.H.F. (2008) Small-scale variation within a Modiolus modiolus (Mollusca: Bivalvia) reef in the Irish Sea. III. Crevice, sediment infauna and epifauna from targeted cores. Journal of the Marine Biological Association UK 88(1): 151-156. Rice, M.A., Valliere, A. & Caporelli, A. (2000) A review of shellfish restoration and management projects in Rhode Island. Journal of Shellfish Research 19: 401-408. Richardson, C.A., Crisp, D.J. & Runham, N.W. (1979). Tidally deposited growth bands in the shell of the common cockle Cerastoderma edule (L.).Malacologia 18: 277-290. Roberts, C. D. (1975) Investigations into a M. modiolus (L.) (Mollusca: Bivalvia) community in Strangford Lough, Northern Ireland. Report, Underwater Association 1:27-49. Roberts, D. (2003) Strangford Lough Ecological Change Investigation. Work Package 2 – The current status of Strangford Modiolus. KA 2.1: Diving Survey. Queen‟s University Belfast. Available from: http://www.doeni.gov.uk/niea/modiolus_position_report22dec03.pdf Roberts, D., Davies, C., Mitchell, A., Moore, H., Picton, B., Portig, A., Preston, J., Service, M., Smyth, D., Strong, D. and Vize, S. (2004) Strangford Lough Ecological Change Investigation (SLECI). Report to Environment and Heritage Service. The Queen's University of Belfast, Belfast. Rowell, T.W. (1967). Some aspects of the ecology, growth and reproduction of the horse-mussel, Modiolus modiolus. M. Sc. Thesis, Queen‟s University Ontario, 138 pp. Sagarin, R.D. & Gaines, S.D., (2002) The „abundant centre‟ distribution: to what extent is it a Biogeographical rule? Ecology letters 5: 137-147. Sanderson W.G., Holt R.H.F., Ramsay K., Perrins J., McMath A.J., and Rees E.I.S. (2008) Small-scale variation within a Modiolus modiolus (Bivalvia) reef in the Irish Sea. Ii. Epifauna recorded by divers and cameras. Journal of the Marine Biological Association UK 88: 143-149 Schulte, D.M., Burke, R.P. & Lipcius, R.N. (2009). Unprecedented restoration of a native oyster metapopulation. Science 325: (5944) 203 Schweinitz, E.H. and Lutz, R.A. (1976) Larval development of the northern horse mussel, Modiolus modiolus (L.), including a comparison with the larvae of Mytilus edulis L. as an aid in planktonic identification. Biological Bulletin 150(3), 348-360. Seddon, P.J., Armstrong, D.P., Maloney, R.F. (2007) Developing the science of reintroduction biology. Conservation Biology 21: 303-312. Seed, R. (1969) The ecology of Mytilus edulis L. (Lamellibranchiata) on exposed rocky shores. I. Breeding and settlement. Oecologia 3 : 277-316 Seed, R. (1976) Ecology. In: Marine Mussels: their Ecology and Physiology (ed. B.L. Bayne), pp. 13-65. Cambridge University Press, Cambridge. Seed, R. and Brown, R.A. (1975) The influence of reproductive cycle, growth, and mortality on population structure in Modiolus modiolus (L.), Cerastoderma edule (L.) and Mytilus edulis L., (Mollusca:Bivalvia). In: H. Barnes (Ed.), 9th European Marine Biology Symposium. Aberdeen University Press, City: 257-274. Seed, R. B., and Brown, R. A. (1977). A comparison of the reproductive cycles of Modiolus modiolus (L.), Cerastoderma (=Cardium) edule (L.), and Mytilus edulis L. In Strangford Lough, Northern Ireland. Oecologia 30:173-188. Seed, R. and Brown, R.A. (1978) Growth as a Strategy for Survival in two Marine Bivalves, Cerastoderma edule and Modiolus modiolus. The Journal of Animal Ecology 47(1): 283-292. Seed R and Suchanek T H. (1992) Population and community ecology of Mytilus. In: Gosling E (editor) The mussel Mytilus: Ecology, Physiology, Genetics and Culture. Elsevier, Amsterdam. pp 87-169. Sergio, C., Figueira, R., Draper, D., Menezes, R., Sousa, A.J. (2007). Modelling bryophyte distribution based on ecological information for extent of occurrence assessment. Biological Conservation 135: 341-351. Service, M. (1990). The impact of commercial trawling on the benthos of Strangford Lough (interim report) Report to Department of Agriculture and Rural Development. Belfast, UK. Service, M. (1998). Monitoring benthic habitats in a marine nature reserve. Journal of Shellfish Research 17 (5): 1487-1489. Service, M. and Magorrian, B. H. (1997). The extent and temporal variation of disturbance to epibenthic communities in Strangford Lough, Northern Ireland. Journal of Marine Biological Association UK 77: 1151-1164. 204 Service, M., Moore H.M, Ferreira, J.S.and Hawkins, A.J.S., in prep. Modelling scenarios for Modiolus modiolus restoration in Strangford Lough. Smyth, D., (2007) Impacts of stock-enhancement strategies on Ostrea edulis in Strangford Lough. Unpublished PhD thesis, Queen’s University Belfast, 233pp. Smyth, D., Roberts, D. and Browne, L. (2009). Impacts of unregulated harvesting on a recovering stock of native oysters (Ostrea edulis). Marine Pollution Bulletin 58 (6): 916-922. Strayer, D.L. (2008). Freshwater Mussel Ecology: A Multifactor Approach to Distribution and Abundance. Freshwater Ecology Series, University of California Press, Berlin. Strong, J. A. and Service, M. (2008) Historical chronologies of sedimentation and heavy-metal contamination in Strangford Lough, Northern Ireland. Biology and Environment: Proceedings of the Royal Irish Academy 108B: 109-126. Tendel, O.S. & Dinesen, G.E. (2005).Biogenic sediments, substrates and habitats of the Faeroese shelf and slope. BIOFAR Proceedings 2005, 224-242. Trimble, A.C., Ruesink, J.L. & Dumbauld, B.R. (2009). Factors preventing the recovery of a historically overexploited shellfish species, Ostrea lurida Carpenter 1864. Journal of Shellfish Research 28(1): 97-106. Utting, S. D. (1988). The growth and survival of hatchery reared Ostrea edulis L. spat in relation to environmental conditions at the on-growing site. Aquaculture 69: 2738. Utting, S.D. & Spencer, B.E. (1991) The hatchery culture of bivalve mollusc larvae and juveniles. Lab. Leafl., MAFF Fish. Res.,68, 31 pp. Veale, L. O., Hill, A. S., Hawkins, S. J., Brand, A.R. (2000). Effects of long-term physical disturbances by commercial scallop fishing on subtidal epifauna assemblages. Marine Biology 137: 325-337. Wesson, J.A., Mann, R & Luckenbach, M.W. (1999). Oyster reef restoration efforts in Virginia In: Luckenbach, M.W., Mann, R., Wesson, J.A. (Eds.), Oyster Reef Habitat Restoration: A Synopsis and Synthesis of Approaches. Virginia Institute of Marine Science Press, Gloucester Point, VA, 117–129. Wiborg, F.K. (1946) Undersøkelser over oskellet (Modiolus modiolus (L.)). Fiskeridirektoratets Skrifter (ser. Havundsrsøkelser), 8, 85. 205 Wildish, D.J., Fader, G.B.J., Lawton, T. & MacDonald, A.J. (1998). The acoustic detection and characteristics of sublittoral bivalve reefs in the bay of Fundy. Continental Shelf Research 18: 105-113. Williams, G. (1954). Fauna of Strangford Lough and neighbouring coasts. Proceedings of the Royal Irish Academy 56: 29-133. Williamson, H.C. (1907) The spawning, growth and movement of the musel (M.edulis L.), horse mussel (M. modiolus L.), and the spoutfish (Solen siliqua L.). Annu. Rep. Scot. Fish. BD. Sci. Invest., 25: 221-255 Wilson, C.D. & Roberts, D. (2011). Modelling distributional trends to develop management strategies for endangered species. Diversity and Distributions 17: 182-189. Wilson, C.D., Roberts, D. & Reid, N. (2011). Applying species distribution modelling to identify areas of high conservation value for endangered species: A case study using Margaritifera margaritifera (L.). Biological Conservation 144: 821-829. Wolf, C.M., Griffith, B. Reed, C. & Temple, S.A. (1996). Avian and mammalian translocations: update and reanalysis of 1987 survey data. Conservation Biology 10:1142-1154. 206 10.0 Appendices 207 Appendix 1. List of Remotely Operated Vehicle (ROV) surveys carried out in Strangford Lough by the Modiolus Restoration Research Group (MRRG) over the 2008 to 2010 period as part of the Modiolus Restoration Plan (MRP) mapping undertaking. Condition grades: 1) Continuous clumps; 2) Discrete clumps; 3) Present; 4) Absent Date 10/04/2008 10/04/2008 10/04/2008 10/04/2008 15/04/2008 22/04/2008 15/04/2008 Northing 54.39343 54.40325 54.40603 54.39943 54.40265 54.40363 54.40840 Easting M. modiolus bed description -5.59883 No evidence of Modiolus -5.61525 No evidence of Modiolus -5.61452 No evidence of Modiolus -5.58533 -5.60325 -5.59150 -5.59200 No evidence of Modiolus No evidence of Modiolus Shell accumulations (80%) Shell accumulations (80%) Condition grade Substrate description Algae/Epifauna 4 Coarse gravel with mixed shell and cobbles Lithothamnion 4 Mixed gravel and shell with occasional small boulders Lithothamnion 4 Coarse sand, gravel, shell and pebbles. Cerianthus lloydii 4 Coarse sand and mixed shell with interdispearsed large boulders Antedon bifida 4 Gravel with frequent large and small boulders Phycodrys rubens 4 Fine sand with overlying gravel and shell including Modiolus Ophiotrix fragilis 4 Fine sand with overlying gravel of which a large proportion are shell Ophiotrix fragilis Henricia oculata 15/04/2008 54.41300 -5.58787 No evidence of Modiolus 4 Gravel overlying fine sand with occasional small and large boulders 22/04/2008 54.41407 -5.62380 No evidence of Modiolus 4 Fine sand Hydroids, Turritella 4 Continuous shell bed (large proportion Modiolusunclear if dead or al Ophiotrix fragilis 15/04/2008 54.41177 -5.58557 Shell accumulations (70%) 208 4 Fine sand with overlying gravel with occasional small and large boulde 4 Fine sand Turritella communis Sagartia elegans 15/04/2008 54.41262 -5.60985 Shell accumulations (70%) 22/04/2008 54.42300 -5.62673 No evidence of Modiolus -5.60995 Shell accumulations (60%) 4 Sand, shelly debris with underlying mud -5.59605 Shell accumulations (60%) 4 Mud and gravel, dead Modiolus Pecten maximus maximus 4 Coarse sand with broken shell with occasional boulders and bedrock out Ensis spp. 4 Soft mud overlaying sandy substrate with no epifauna evident No apparent epifauna 2 sandy and mud with dead and living Modiolus Antedon bifida -5.61007 Shell accumulations (80%) 4 Sand and gravel with dead Modiolus shell and Nephrops burrows Thyone sp. -5.60997 No evidence of Modiolus 4 Sand and mud Turritella -5.60008 No evidence of Modiolus 4 Muddy with some overlaying gravel Carcinus maenas Virgularia mir 16/04/2008 16/04/2008 16/04/2008 16/04/2008 16/04/2008 16/04/2008 16/04/2008 16/04/2008 54.42267 54.42257 54.42213 54.43030 54.43010 54.43208 54.44222 54.44190 -5.58560 -5.60255 -5.59630 No evidence of Modiolus No evidence of Modiolus Small clumps Lithothamnion 16/07/2008 54.43597 -5.58605 No evidence of Modiolus 4 Mud with Nephrops burrows and frequent Sagartia elegans and occasional 22/04/2008 54.43597 -5.58605 No evidence of Modiolus 4 Fine sand Thyonidium drumondrii 21/04/2008 54.44193 -5.58725 No evidence of Modiolus 4 Fine sand with overlying gravel No apparent epifauna -5.60720 Shell accumulations (40%) 4 Vast quantities of Modilus shell. Asterias rubens 4 Fine sand with dense coverage of shell - Alive and dead with possible Modiolus 4 Fine sand with overlying gravel (shell debris) Turritella 21/04/2008 22/04/2008 21/04/2008 54.45082 54.45082 54.46108 -5.57657 Shell accumulations (40%) -5.59785 No evidence of Modiolus 209 21/04/2008 22/04/2008 21/04/2008 21/04/2008 21/04/2008 21/04/2008 21/04/2008 21/04/2008 21/04/2008 21/04/2008 21/04/2008 54.46072 54.46070 54.46885 54.46890 54.47620 54.48490 54.49108 54.49105 54.50145 54.50178 54.50855 -5.58183 Shell accumulations (60%) -5.58182 Shell accumulations (70%) -5.59807 No evidence of Modiolus -5.58650 -5.59862 -5.59985 -5.61233 -5.59902 -5.61290 Shell accumulations (80%) No evidence of Modiolus No evidence of Modiolus No evidence of Modiolus No evidence of Modiolus No evidence of Modiolus Thyone spp. 4 Fine sand with broken shells and occasional clumps of shell possibly M Antedon bifida 4 Fine sand with overlying gravel (shell debris) 4 Lots of shells including Modiolus valves with rich epifauna dominated Calliostoma sp. 4 Mud with overlying sand with occasional epifauna Urticina eques 4 Mud with Nephrops burrows and no obvious epifauna Nephrops 4 Mud with Nephrops burrows and no obvious epifauna Asterias rubens 4 Mud with Nephrops burrows and no obvious epifauna No apparent epifauna 4 Fine sand with abundant overlying shell and pebbles Hydroids 4 Fine sand with coarse gravel including shell debris Henricia oculata 4 Fine sand with plentiful shell debris Hydroids No apparent epifauna No evidence of Modiolus -5.60067 No evidence of Modiolus 4 Fine sand with overlying shell debris (cockles) 4 Cobbely gravel Hydroids,Pecten maximus 2 Fine sand / mud with overlying scattered shell debris covered by super Asterias rubens 21/04/2008 54.51630 -5.61738 23/04/2008 54.41837 -5.61012 No evidence of Modiolus 54.42075 To be repeated -5.60130 No evidence of Modiolus 23/04/2008 4 -5.62283 Small clumps 210 23/04/2008 23/04/2008 23/04/2008 23/04/2008 54.42102 54.42122 54.42102 54.42272 -5.61877 Shell accumulations (60%) -5.61340 No evidence of Modiolus -5.60995 Small clumps -5.60340 Shell accumulations (40%) 4 Mud to fine sand with superficial silt on all surfaces Asterias rubens 4 Mud / fine sand with Nephrops burrows Turritella 2 Fine sand with dense overlying debris of scattered dead and occasioa Asterias rubens 4 Fine sand with scattered shell debris Crossaster paposus Fine sand with overlying shell and occasional cobbles. Small living c Echinus esculentus 23/04/2008 54.42353 -5.61840 Small clumps 2 23/04/2008 54.42360 -5.61340 No evidence of Modiolus 4 Fine sand Turritella 4 Fine sand with sparse epifauna Turritella 2 Mud Modiolus Asterias rubens 23/04/2008 54.42547 -5.62270 No evidence of Modiolus 23/04/2008 54.42550 -5.61822 Small clumps 4 Fine sand with scattered shell debris 4 tide too strong,to be repeated 4 Muddy sand with overlying hydroids and polychaeta Asterias rubens 2 Shelly gravel mix Modiolus Carcinus naena 23/04/2008 54.42550 -5.61337 No evidence of Modiolus 23/04/2008 54.42543 -5.61007 No evidence of Modiolus 25/04/2008 25/04/2008 23/04/2008 23/04/2008 54.42543 54.42528 54.42962 54.42848 -5.61007 No evidence of Modiolus -5.60472 Small clumps -5.62495 Shell accumulations (30%) 4 Fine sand with overlying gravel, pebbles and cobbles. -5.61695 Shell accumulations (50%) 4 Muddy substrate with shell debris. Antedon bifida Nephrops norve 23/04/2008 54.42853 -5.61333 Small clumps 2 Muddy substrate with Nephrops burrows and overlying shelly debris. 25/04/2008 54.42847 -5.61003 Small clumps 2 Shelly mud Modiolus -5.60490 No evidence of Modiolus 4 Mud and Nephrops burrows Turritella -5.60022 Shell accumulations (80%) 4 Continuous dead shell debris Alcyonium digitatum 25/04/2008 01/05/2008 54.42837 54.44808 211 01/05/2008 01/05/2008 01/05/2008 01/05/2008 01/05/2008 01/05/2008 01/05/2008 01/05/2008 01/05/2008 01/05/2008 01/05/2008 01/05/2008 54.44803 54.45068 54.45067 54.45283 54.45278 54.45525 54.45512 54.45510 54.45758 54.45748 54.45767 54.44518 -5.58783 Shell accumulations (70%) 4 Gravel with cobbles and empty shells Asterias rubens -5.60017 Shell accumulations (90%) 4 Continuous shelly debris with a silty coverage Asterias rubens 2 Fine sand with abundant overlying shell Modiolus -5.59478 Shell accumulations (70%) 4 Fine sand with overlying shelly debris and moderate epifauna Ophiocomina nigra -5.58817 Shell accumulations (60%) 4 Mud with overlying shell Cliona celata -5.59995 Shell accumulations (50%) 4 Mud with overlying gravel and empty shells Hydroids,Antedon 2 Fine sand with dense cover of shells Modiolus modio 2 Fine sand with overlying dense cover of shell50% of which is Modiolu Henricia spp., Liocarcinus sp. de -5.58835 -5.59465 Small clumps Small clumps -5.58823 Small clumps -5.59477 Shell accumulations (80%) 4 Fine sand with dense over lying shelly debris -5.58810 Shell accumulations (70%) 4 Shelly mud. Liocarcinus sp. sp 4 Fine sand with overlying gravel and shelly debris with occasional larg Henricia spp., 4 Fine sand with overlying dense shelly debris Thyone sp. fusus Modiolus modio -5.60158 Shell accumulations (80%) -5.59987 Shell accumulations (80%) 02/05/2008 54.44500 -5.58772 Small clumps 2 Sandy substrate high shell density 06/05/2008 54.46033 -5.60602 No evidence of Modiolus 4 Fine sand -5.58822 Shell accumulations (80%) 4 Dense broken shell None 4 Fine sand with overlying shelly debris and silt coverage on all surfac Asterias rubens 4 Sandy bottom with sparse shelly debris Poor, barren s 02/05/2008 02/05/2008 02/05/2008 54.46072 54.45733 54.45490 -5.58230 Shell accumulations (80%) -5.58245 No evidence of Modiolus 212 02/05/2008 54.45268 -5.58283 Shell accumulations (70%) 02/05/2008 54.45050 -5.58302 occasional Modiolus 3 Muddy sand Modiolus 02/05/2008 54.44782 -5.58297 Small clumps 2 Shelly sand Modiolus 02/05/2008 54.44510 -5.58323 Small clumps 2 Fine sand Modiolus -5.57735 Shell accumulations (70%) 4 Fine sand and some silt Antedon bifida 4 Mud with overlying dead shell Ophiocomina ni 02/05/2008 54.44202 4 Shell on sandy bottom 25/04/2008 54.45050 -5.59013 No evidence of Modiolus 06/06/2008 54.45490 -5.57633 No evidence of Modiolus 4 Sand (95%), 5% broken shell Sparse epifaun 06/06/2008 54.46047 -5.56548 No evidence of Modiolus 4 90% fine sand, 10% empty shell No apparent ep 06/06/2008 54.46568 -5.56078 No evidence of Modiolus 4 80% fine sand, 20% empty shell No apparent ep -5.55377 No evidence of Modiolus 4 70% sand, 30% broken shell No apparent ep 4 Fine sand with some shell. Anemones common 4 50% sand, 50% cockle shell A. rubens (F) 4 60% Shell, 40% fine sand and a layer of silt on all substrata Thyone sp. Thyone sp. 06/06/2008 54.47028 06/06/2008 54.47912 -5.57145 No evidence of Modiolus 06/06/2008 54.47633 -5.57602 No evidence of Modiolus -5.58612 Shell accumulations (60%) 4 40% fine sand , 60% shelly debris 4 Mud and fine sand Algal biofilm Marthasterias, 06/06/2008 54.47585 06/06/2008 54.47270 -5.58790 Shell accumulations (70%) 09/06/2008 54.46657 -5.57133 No evidence of Modiolus 09/06/2008 54.39703 -5.61803 Small clumps 2 Gravelly dead shells 09/06/2008 54.39928 -5.58998 No evidence of Modiolus 4 Gravelly, cobble, pebble, boulders Ophiocomina nigra 4 Boulders, gravel, dead shell and cobble Ophiocomina nigra 4 Shelly sand and Boulders Nemertesia, Ophiurids 4 Selly sand with some empty Modiolus Ophiotrix bed Ophiocomina ni 09/06/2008 54.40220 -5.59667 Shell accumulations (60%) 09/06/2008 54.40612 -5.59237 No evidence of Modiolus -5.59468 No evidence of Modiolus -5.60902 Shell accumulations (90%) 4 Empty shell/gravel, Boulders -5.59788 Shell accumulations (60%) 4 Fine sand and shells Ophiocomina ni -5.58590 No evidence of Modiolus 4 Fine shelly sand and some empty shells Hydroids,Ophiu 09/06/2008 09/06/2008 09/06/2008 09/06/2008 54.41233 54.41585 54.41787 54.41800 213 09/06/2008 09/06/2008 10/06/2008 10/06/2008 54.42753 54.42662 54.46167 54.46817 -5.58233 Shell accumulations (90%) 4 Dead shell and gravel Sagartiogeton, -5.59608 No evidence of Modiolus 4 Shelly sand Hydroids, Saga -5.60938 Shell accumulations (50%) 4 Shell substrate on mud or dine sand bottom Somw red algae -5.61377 Shell accumulations (50%) 4 Shell on fine sand with odd patch of Nephrops ground Sparse epifaun 4 Moderate amounts of shell Shrimp, Thyone sp. 10/06/2008 54.47293 -5.59815 Shell accumulations (90%) 10/06/2008 54.46447 -5.59642 No evidence of Modiolus 4 Mud/ fine sand Nephrops, Asterias 10/06/2008 54.47400 -5.61300 No evidence of Modiolus 4 Shell debris on mud with silt Hydroids, Nephrops Pagurus sp. 10/06/2008 54.48083 -5.61410 No evidence of Modiolus 4 Empty shells, muddy sand, Nephrops burrows 10/06/2008 54.48305 -5.60725 No evidence of Modiolus 4 Shell debris on Mud - very silty Nephrops 13/06/2008 54.43628 -5.57755 No evidence of Modiolus 4 Muddy sandy Gobius sp., hydroids 13/06/2008 54.44730 -5.57688 No evidence of Modiolus 4 Pebble, gravel Antedon bifidañ Gobius sp. 13/06/2008 54.43685 -5.59303 No evidence of Modiolus 4 Mud Turritella, Nephrops 13/06/2008 54.43718 -5.61057 4 Muddy sand Nephrops 13/02/2009 54.42847 -5.59902 No evidence of Modiolus High density of clumps (510) with patches of Modiolus shell 1 Hydroid turf Small clumps -5.59364 fine silty sand between patches of brittle stars 4 Dense O. fragilis, Echinus No evidence of Modiolus -5.59891 Moderate Modiolus shell on shelly mud 2 13/02/2009 13/02/2009 54.42846 54.43041 13/02/2009 54.43048 -5.59375 13/02/2009 54.43235 -5.59874 13/02/2009 54.43239 -5.59364 small frequent clumps on silty fine sand fine silty sand with occasional Modiolus shell Frequent clumps (5-10) on silty fine sand and dense Modiollus shell 13/02/2009 54.43700 -5.59888 soft muddy sand 1 Accumulations of shell Echinus abundant Small clumps Accumulations of shell 3 1 Many crinoids on clumps Small clumps 4 Nephrops and Turritella No evidence of Modiolus 214 Silty fiine sand and mud 4 no epifauna, Nephrops No evidence of Modiolus 4 Nephrops and Asterias No evidence of Modiolus 2 Nephrops , cerianthus Accumulations of shell 1 Abundant epifauna on clumps:Antedon, Hydroids, O. nigra, E.esculentus, Flustra foliacea Very large clumps (>10 individuals) -5.59106 Distinct individual occasional small clumps (1-4) on a sandy substrate 2 Sparse epifauna: Echinus esculentus, Hydroids, Solaster paposus, Alcyonium Small clumps -5.59888 Muddy sand with broken shell and occasional cobble 4 Nephrops No evidence of Modiolus Frequent clumps (5-10) dense with occasional barren areas on fine sand and silt with broken shell 1 Asterias, Antedon, More mobile epifaunal species Very large clumps (>10 individuals) 13/02/2009 54.44138 -5.59247 13/02/2009 54.44379 -5.59684 13/02/2009 54.44409 -5.59149 Shelly muddy sand Small clumps of shell on silty fine sand with occasional cobbles -5.59634 Large very frequent clumps (1-6) on coarse sand and shell mix (10% shell) 16/02/2009 16/02/2009 13/02/2009 54.43040 54.43053 54.43495 13/02/2009 54.43509 -5.59378 13/02/2009 54.43817 -5.59303 13/02/2009 54.42661 -5.60393 Fine silty sand with Nephrops Silty fine sand with a little mud with some broken shell on surface 13/02/2009 54.42446 -5.59928 13/02/2009 54.42312 -5.60057 Continuous patches of broken shell and cobbles Shell on cobble and pebble 16/02/2009 54.43052 -5.58774 Sandy mud shell mix 16/02/2009 16/02/2009 54.43246 54.43544 -5.58835 -5.58962 Occasional clumps of Modiolus on sandy shell Large clumps in close proximity frequent (1-8) on sandy mud with shell mix 4 No evidence of Modiolus 4 Accumulations of shell 4 No evidence of Modiolus 4 No evidence of Modiolus 4 sparse: Ceriantus lloydii Accumulations of shell 2 Sparse: Echinus Small clumps 1 Antedon, O.nigra, E. esculentus, Hydroids, Asterias Very large clumps (>10 individuals) 215 16/02/2009 54.43815 -5.58918 16/02/2009 54.42681 -5.59895 16/02/2009 16/02/2009 54.42653 54.43245 -5.59330 -5.59090 Occasional small clumps on 80% coverage of dead Modiolus shell Sandy/mud with dead Modilus, Chlamys and Ostrea mix Frequent small clumps (1-5) on coarse sand/shell (Modiolus and Chlamys) Frequent clups (1-8) with some large clumps (12+)on sandy shell with broken Modiolus and Chlamys 16/02/2009 54.43554 -5.58667 17/02/2009 54.42479 -5.59316 Sandy substrate with some ripples Frequent small (1-4) with occasional large clump on shelly sandy mud. Dense accumulations of Modiolus shell -5.58741 Boulder substrate unlevelled ground filled with shell and silt 16/02/2009 17/02/2009 54.43751 54.42473 -5.59408 17/02/2009 54.42661 -5.59074 17/02/2009 54.42853 -5.59090 17/02/2009 54.42869 -5.58783 Large boulder piles with area of cobble Occasional clumps of Modiolus (810) on sandy with shell substrate Sandy with 20% shell debris 17/02/2009 54.42856 -5.58453 17/02/2009 54.43055 17/02/2009 54.43425 4 Sparse: Solaster, Thyone sp., Antedon Cerianthus lloydii, Echinus Esculentus and Pecten maximus maximus Accumulations of shell 1 sparse epifauna with Echinus esculentus Small clumps 1 Antedon, Echinus Hydrois Asterias O. nigra, Solaster paposus Large clumps 4 Urticina eques No evidence of Modiolus 2 No antedon on clumps. Sparse epifauna Small clumps 4 No epifauna Accumulations of shell 4 Antedon, Pecten maximus, Hydroids, Echinus No evidence of Modiolus 4 abundant assemblages on boulders No evidence of Modiolus 2 2 Small clumps Large clumps 4 Nephrops , cerianthus Accumulations of shell Modiolus shell on cobble 4 Hydroids Accumulations of shell -5.58489 Soft mud 4 No evidence of Modiolus -5.58477 Sandy mud on cobbles 4 No evidence of Modiolus 216 17/02/2009 17/02/2009 17/02/2009 17/02/2009 17/02/2009 18/02/2009 18/02/2009 18/02/2009 18/02/2009 28/04/2009 54.43397 54.43399 54.43915 54.44206 54.42492 54.44202 54.44044 54.44415 54.44584 54.51623 -5.58703 -5.58942 Occasional small clumps (1-5) on silty sand with shell mix Frequent large clumps (10+) on silty sand 2 Hydroids Small clumps 1 O. nigra, Antedon, Hydoids Large clumps 4 Sparse epifauna No evidence of Modiolus 1 well developed Very large clumps (>10 individuals) Amphiura, Pecten maximus Hydrois, Flustra No evidence of Modiolus -5.58208 Muddy sand with broken shell and occasional cobble Frequent small clumps (1-5) and large (15+) in high densities on silty sand. Looks like a fragmented bed -5.59044 Cobble on fine sand with shell debris 4 -5.57936 Sandy muddead Modiolus bed 4 -5.57966 Shelly mud ( Modiolus) on boulder outcrops and bed rock 4 sparse epipfauna Accumulations of shell -5.58162 Frequent clumps (1-10) individuals) 1 Antedon, hydroids, echinus abindant. Very large clumps (>10 individuals) Accumulations of shell -5.57887 Accumulations of shell -5.57775 Coarse shell mix 4 Antedon, abundant hydroids, Nemertesia, S.argentia, Abundant echinus -5.62313 Soft mud, no apparent epifauna 4 Soft Mud None apparent 4 Mud with some shell (not Modiolus) N. norvegicus 4 soft mud None apparent 4 soft mud None apparent 4 soft mud None apparent 4 soft mud None apparent 4 soft mud None apparent 4 soft mud None apparent 4 soft mud None apparent 4 mud Hydroids 28/04/2009 54.51558 -5.62132 28/04/2009 54.51845 -5.61157 28/04/2009 54.51840 -5.61750 28/04/2009 54.51865 -5.62232 28/04/2009 54.51867 -5.62747 28/04/2009 54.52065 -5.62752 28/04/2009 54.52083 -5.62235 28/04/2009 54.52080 -5.61758 Nephrops ground None apparent None apparent None apparent None apparent None apparent None apparent None apparent 11/05/2009 54.45742 -5.56938 None 217 11/05/2009 11/05/2009 11/05/2009 54.46305 54.46477 54.46973 -5.56248 10% shell 4 very soft A. abietina, Iophon hynmandi, A.rubens, Myxilla, Cliona celata -5.55695 5% shell on Nephrops ground 4 very soft Nephrops norvegicus -5.55932 Nephrops burrows 4 very soft mud with 70% mixed shell 4 4 80% crushed shell C. lepadiformis, C. celata 4 broken shell on soft mud hydroids, sponges, abietinaria 4 soft mud, scattered shell S. elegans, E. esculentus 4 soft mud N. norvegicus 4 soft mud S. elegans 11/05/2009 54.47152 -5.57060 Soft sediment with sponges and hydroids 11/05/2009 54.47242 -5.56972 Firmer than previous sites Soft mud with clumps of hydroids Soft bottom with scattered shell 11/05/2009 54.47402 -5.56783 11/05/2009 54.47627 -5.56530 11/05/2009 54.47968 -5.56187 11/05/2009 54.47968 -5.56187 Nephrops ground Soft mud with some broken shell on surface 20/05/2009 54.51133 -5.61527 None 4 very soft sand None apparent 20/05/2009 54.51192 -5.61283 None 4 very soft sand None apparent A.rubenss, hydroids 20/05/2009 54.50883 -5.60330 None 4 mixed shell (50%) not Modiolus and occasional Nephrops burrows 20/05/2009 54.51185 -5.61262 5% Modiolus shell 4 Hydroid clumps 20/05/2009 54.50568 -5.61460 40% Modiolus shell 4 Soft with shell and overlying silt 4 Sofft undulating substrate with shell 96%) OF cockle size 4 Mixed sustrate : cobbles (70%)soft sediment with Hydroid turf covering surfaces 4 very soft bumpy sediment with 50% shell (subfossil) 4 very soft bumpy sediment with 50% shell (subfossil) 20/05/2009 20/05/2009 20/05/2009 20/05/2009 54.50182 54.49737 54.49840 54.49923 -5.60995 -5.60285 -5.60907 -5.60075 None None None None C.paposus Hydroids, A.spirodeta, A digitatum 218 20/05/2009 20/05/2009 20/05/2009 54.49822 54.49415 54.49415 -5.59753 -5.59598 -5.60153 None None None 4 Nephrops Ground 4 very soft bumpy sediment with 50% shell (subfossil) 4 very soft bumpy sediment with 50% shell (subfossil) Nephrops norvegicus 20/05/2009 54.49415 -5.60663 None 4 very soft bumpy sediment with 50% shell (subfossil) 20/05/2009 54.49415 -5.59500 None 4 Nephrops Ground Nephrops norvegicus 20/05/2009 54.49138 -5.59085 None 4 Nephrops Ground Nephrops norvegicus 20/05/2009 54.49203 -5.59500 None 4 sub-fossil Oyster shell None 20/05/2009 54.48753 -5.59610 None 4 sub-fossil Oyster shell None 4 sub-fossil Oyster shell and some Nephrops burrows None 4 Soft shelly mud with boulders and Nephrops A. digitata, Metridium senile, Hydroids 4 Shelly fine sand with Scallop shell and Nephrops burrows Hydroids, Liocarcinus sp. and N. norvegicus 4 Shelly fine sand with Nephrops burrows M. senile, Liocarcinus sp. nad Nephrops 4 Shell fragments on muddy fone dand Carpet of Hydroids, occasional sponge (Amphilectus sp.) and S. cillata 4 Shell fragments on mud with Nephrops burrows N. norvegicus, Hydroids and Anemones 4 Hydroids and shell on muddy sand Scallops, Liocarcinus sp. 4 Shelly fine sand with hydroids N. norvegicus, U. eques, Liocarcinus sp. 4 Shelly muddy sand with Mounds and Nephrops Burrows N. norvegicus and A. rubens 20/05/2009 21/05/2009 21/05/2009 21/05/2009 21/05/2009 21/05/2009 21/05/2009 21/05/2009 21/05/2009 54.48830 54.48675 54.48518 54.48473 54.48245 54.49020 54.47883 54.47878 54.47887 -5.60208 -5.60682 -5.60663 -5.59290 -5.59403 5% None None None None -5.59968 None -5.60535 Abundant Modiolus Shell (>70%) -5.59942 Occasional M.modiolus shell (20%) -5.59478 None 219 21/05/2009 54.47935 4 Shell debris nad Hydroid cover on muddy sand 4 Muddy sand 4 Shell fragments abundant on muddy sand with hydroid cover on a flat bottom Hydroids, Liocarcinus sp. 4 Soft shelly muddy sand with occasional Modiolus shell Hydroids, Liocarcinus sp., Thyone sp., Sponges 4 Soft shelly muddy sand with occasional Modiolus shell Crangon, Amphilectus, U. eques, A. rubens, Thyone sp., M. senile, Pecten maximus 4 resembled old M. modilus bed on muddy sand with Scallop shell C. lepadiformis, Liocarcinus sp., Sponges, Anemone, A.rubens, Thyone sp. Hydroids, Liocarcinus sp. 54.47603 -5.59328 21/05/2009 54.47537 -5.60528 None 21/05/2009 21/05/2009 21/05/2009 21/05/2009 54.47363 54.47070 54.47063 54.47057 M. senile, Liocarcinus sp., U.eques, T. fusus None 21/05/2009 54.47272 hydroids -5.58922 Some Modiolus shell (10%) 21/05/2009 4 Shell fragments on muddy sand with hydroid cover -5.60408 None -5.58880 Some Modiolus shell (10%) -5.58757 -5.58260 some Modiolus shell (10%) Abundant Modiolus shell (70%) -5.59807 None 4 Shell fragments on muddy sand with Hydroids Very fine sand and soft mud, Nephrops ground 4 no apparent species 4 no apparent species 22/05/2009 54.46643 -5.59783 23/05/2009 54.46825 -5.58990 24/05/2009 54.46727 -5.58580 25/05/2009 54.45927 -5.58698 Modiolus and Chlamys shell Abundant shell Abundant shell 26/05/2009 54.46660 -5.58182 Odd Modiolus shell 4 4 Mud 4 Fine sand/mud A. Bifida, O. Fragilis, Hydroids, P. Maximus, Sponges, S. Paposus 02/06/2009 54.41608 -5.60225 None 4 gravelly sand with small boulders, cobble & pebbels (40%) 22/06/2009 54.41248 -5.60623 O. fragilis bed 4 gravel, sand O. Fragilis 22/06/2009 54.41445 -5.60248 O. fragilis bed 4 boulders on gravel O. Fragilis -5.60782 None apparent 4 muddy sand with shell 22/06/2009 54.49825 220 22/06/2009 22/06/2009 22/06/2009 22/06/2009 54.49840 54.49810 54.40983 54.40758 -5.61327 None apparent -5.59510 Muddy sand with some shell debris -5.62638 Turritella, brittle star arms muddy sand with some cobbles/pebbleb (10%) Echinus, Sycon, Liocarcinus sp., burrowing anemones muddy sand Turritella, anemones, brittle stars 4 Fine sand/mud Turritella, anemones, Necora puber 4 4 -5.63218 None apparent 4 mud with some coblle and bedrock outcrops 4 shelly coarse sand Flustra foliacea 4 gravel, boulders O. fragilis 22/06/2009 54.40852 -5.61145 None apparent 22/06/2009 54.40878 -5.60172 O. fragilis bed 4 boulders on cobbles, pebbel, shell 22/06/2009 54.40305 -5.60885 Anemone covered boulders 22/06/2009 54.40617 -5.60823 Shell aggregations 4 Dense shell on gravel 22/06/2009 54.40605 -5.60262 None apparent 4 boulders on shelly gravel -5.60662 None apparent 4 shelly gravel with patchces of fine sand 4 coarse sandy bottom with Occasional A. rubens and occasional O.fragilis clumps 4 coarse sand with patches of O. fragilis 4 fraquent sma;; boulders with high coverage of epifauna O.fragilis, hydroids, Urchins and Oysters 4 Boulders of mixed sizes with cobbles and gravel and thick coverage of O. nigra O.nigra 4 gravelly with occasional boulders O.albida, O.fragilis, O. nigra 4 Coarse sand with ophiuroids Sparse epifauna with frequent O.albida, 4 fine sand with overlaying broken shell 4 soft mud with occasional large boulders 22/06/2009 12/10/2009 12/10/2009 12/10/2009 12/10/2009 12/10/2009 12/10/2009 12/10/2009 12/10/2009 54.40882 54.41398 54.41398 54.41328 54.41502 54.41482 54.41823 54.41983 54.41983 -5.58928 Very few shell -5.59248 Good shell coverage (70%) -5.59680 -5.59592 -5.58817 -5.58728 -5.59492 -5.59902 Shell Sparse shells shell None apparent Good coverage of M.modiolus shell (60%) O. Fragilis bed with Modiolus shell (30%) Ophiurids 221 12/10/2009 12/10/2009 13/10/2009 54.42100 54.42100 54.44875 4 coarse gravel (mostly shell) overlying fine sand bottom with 60% coverage of MM shell. A. Bifda, A. Aspersa Gravel with overlying Modiolyus shell and O. Fragilis patches O. fragilis -5.59912 Modiolus shell (60%) -5.59525 Modiolus shell (30%)and O. fragilis patches 4 -5.58083 Muddy bottom with frequent Modiolus shell (70%) 4 13/10/2009 54.44853 -5.57592 13/10/2009 54.44693 -5.58190 13/10/2009 54.44672 -5.57937 Gravelly shell with cobbles and pebbles Shelly but with Modiolus shell Cobbles , shell on mud with live clumps (2-5) individuals 4 4 2 13/10/2009 54.44675 -5.57530 13/10/2009 54.44435 -5.57807 13/10/2009 54.43897 -5.57585 13/10/2009 54.43838 -5.58000 Cobbles, shell, mud with overlying Modiolus shell Shelly mud with small fragment of Modiolus shell Muddy sand with some shell ( not Modiolus) live clumps and Modiolus shell on mud, cobble, pebble and small boulders -5.58342 Some shell fragments 4 -5.58345 Some Modiolus shell 4 -5.57897 Occasional shell 4 shelly mud 13/10/2009 13/10/2009 13/10/2009 54.43823 54.43577 54.43592 4 4 4 2 shelly mud 13/10/2009 54.43587 -5.57407 4 muddy sand mounds 13/10/2009 54.43380 -5.57628 4 muddy sand mounds 13/10/2009 54.43377 -5.58073 4 shelly gravel 11/08/2010 54.41647 -5.58907 No evidence of Modiolus 4 Fine sand with crushed shell 11/08/2010 54.41875 -5.58802 No evidence of Modiolus 4 mudd O. Fragils, O.nigra, 222 11/08/2010 54.41838 -5.59103 11/08/2010 54.42142 -5.59210 11/08/2010 54.42142 -5.59153 11/08/2010 54.42158 -5.58895 O. fragilis patch with O. Nigra around outside O. fragilis bed, extensive among boulders no apparent life O. fragilis patches (small) with anemones 11/08/2010 54.43853 -5.59622 Nephrops 4 soft bottom N.norvegicus 11/08/2010 54.43715 -5.60025 Nephrops 4 soft bottom N.norvegicus 11/08/2010 54.43857 -5.60362 Nephrops 4 soft bottom N.norvegicus 11/08/2010 54.44150 -5.60233 O. Fragilis, O. nigra 4 4 mudd, large boulders 4 very soft bottom 4 4 Occasional shell into broken shell 11/08/2010 54.43982 -5.60442 11/08/2010 54.43915 -5.58505 11/08/2010 54.44683 -5.58593 Modiolus shell 4 fine sand 11/08/2010 54.44692 -5.58903 Modiolus shell 4 fine sand 11/08/2010 54.44633 -5.58913 Crushed shell (100%) 4 -5.58655 Shell aggregations (80%) 4 -5.58597 shell aggregations (100%) 4 4 11/08/2010 11/08/2010 54.44748 54.44778 4 fine sand 11/08/2010 54.44792 -5.58568 Shell aggregations finer (80%) 11/08/2010 54.44820 -5.58530 Shell (60%) 4 Fine sand 11/08/2010 54.47403 -5.59240 Old Modiolus shell 4 Fine sand T. roscovita 4 Fine sand 11/08/2010 54.47383 -5.58402 100% crushed shell with A. aspersa mounds 11/08/2010 54.42413 -5.59762 Some shell 4 Fine sand A.aspersa A.aspersa, alcyonium digitata, Pecten maximus maximus, Thyone sp. spp. -5.59960 100% crushed shell with A. aspersa mounds 4 Fine sand A.aspersa 4 Fine sand A.aspersa 4 Fine sand 11/08/2010 54.47458 11/08/2010 54.46438 -5.59108 100% crushed shell with A. aspersa mounds 11/08/2010 54.46210 -5.58535 100% crushed shell 223 Appendix 2. List of dive surveys carried out in Strangford Lough by the Modiolus Restoration Research Group (MRRG) over the 2008 to 2010 period. Northing Easting Max Depth (m) 54.4508 -5.6009 20 54.4508 -5.6009 12 Tasks DPV reef boundary mapping DPV reef boundary mapping 54.4509 -5.6283 17.3 Live Modiolus collection 54.4478 -5.6272 14.2 Date No. dives 17/07/2008 1 17/07/2008 1 22/07/2007 1 22/07/2008 1 Location Scott‟s Hole ( MOD 4) Scott‟s Hole ( MOD 4) Ringhaddy Sound North most hole Ringhaddy Sound middle hole opposite pier 23/07/2008 23/07/2008 1 1 Taggart Island Taggart Island 24/07/2008 1 SE Hadd Rock 54.4550 -5.5956 16.8 24/07/2008 1 SE Hadd Rock 54.4539 -5.5937 21.2 25/07/2008 2 Brown Rocks 54.4241 -5.6203 15 25/07/2008 2 28/07/2008 2 28/07/2008 2 Brown Rocks W of Sand Rock heading south W of Sand Rock heading North 29/07/2008 2 01/08/2008 21/08/2008 22/08/2008 26/08/2008 28/08/2008 28/08/2008 28/08/2008 29/08/2008 08/09/2008 08/09/2008 2 2 2 2 3 1 1 2 1 1 08/09/2008 1 W Sand rock SSE Black Rock S Hadd Rock S Hadd Rock S Hadd Rock S Hadd Rock S Hadd Rock S Hadd Rock S Hadd Rock S Hadd rock S Hadd rock East of Hadd Pole 19.4 12.5 Live Modiolus collection Live Modiolus collection 54.4530 54.4534 54.4530 -5.5917 -5.5914 -5.5917 27.0 23.7 17.6 21.7 Live Modiolus collection Live Modiolus collection Looking for southern good site Looking for southern good site Finding Boundary of Modiolus bed with DPVs Finding Boundary of Modiolus bed with DPVs Finding Boundary of Modiolus bed with DPVs Live Modiolus collection Quadrats Quadrats Quadrats Quadrats Quadrats Quadrats Quadrats Quadrats Quadrats 54.4531 -5.5917 19.8 Quadrats 17.7 12 54.4543 -5.5938 29.2 54.4246 54.4534 54.4534 -5.6176 -5.5914 -5.5914 21 28 18 22.0 224 10/09/2008 2 1 16/09/2008 1 1 16/09/2008 1 2 1 17/09/2008 1 17/09/2008 1 1 1 1 17/09/2008 1 1 17/09/2008 1 1 1 1 18/09/2008 22/09/2008 24/09/2008 1 1 1 1 11 Quoile North of Green Island North Janes rock North Janes rock North Janes rock North Janes rock North Janes rock to Scott‟s hole North Janes rock to Scott‟s hole North Janes rock to Scott‟s hole Scott‟s Hole to Finyan pt Janes rock to sand rock Janes rock to sand rock Heading south from Janes rock in the channel Heading south from Janes rock in the channel 5.8 Quadrats 16 exploratory 54.4501 -5.6030 54.4491 -5.6135 exploratory 54.4501 -5.6030 exploratory 54.4508 -5.6057 54.4508 -5.6057 exploratory 54.4505 -5.6070 exploratory 54.4503 -5.6080 exploratory 54.4504 54.4505 54.4506 -5.6272 -5.6138 -5.6153 exploratory exploratory exploratory 54.4499 -5.6031 exploratory 54.4517 -5.6007 exploratory 54.4464 -5.6017 34.8 exploratory Repeat SLECI transect from Hadd to Slave Hadd rock to Slave 20.8 exploratory South Hadd rock South Hadd rock South Hadd rock (good Modiolus position) swimming west South Hadd rock (good Modiolus) 54.4549 54.4552 -5.5944 -5.5944 24.4 Repeat SLECI site eastward to site of good Modiolus 54.4539 54.4552 -5.5937 -5.5978 20.8 exploratory 54.4539 -5.5937 22.3 Quadrats 225 29/09/2008 1 2/10/20008 1 2/10/20008 1 06/10/2008 2 06/10/2008 07/10/2008 08/10/2008 08/10/2008 2 2 1 1 15/10/2008 3 15/10/2008 1 15/10/2008 1 South Hadd rock (good Modiolus position) South Hadd rock (good Modiolus position) South Hadd rock (good Modiolus position) East of Brown Rock Pladdy East of Brown Rock Pladdy East of Brown Rock Pladdy East of Brown Rock Pladdy East of Brown Rock Pladdy East of Brown Rock Pladdy North of Dunnyneill Colin Rock Marlfield bay Marlfield bay S. Hadd godd site(missed) S. Hadd godd site S. Hadd godd site 03/11/2008 2 1 S.Hadd S.Hadd 54.4555 54.4555 -5.5964 -5.5964 20 20 2 S.Hadd 54.4555 -5.5964 22.3 1 S.Hadd 54.4555 -5.5964 22.3 2 S.Hadd 54.4555 -5.5964 20.5 1 1 1 1 1 1 1 S.Hadd 54.4555 54.4555 54.4235 54.4238 54.4241 54.4272 54.4272 -5.5964 -5.5964 -5.6140 -5.6154 -5.6151 -5.6159 -5.6156 20.1 23.3 35.8 DPV mapping Deploying spat collectors Spat collectors Deploying spat collectors Deploying spat collectors Deploying spat collectors Deploying spat collectors Exploratory Exploratory 27.9 Exploratory 1 2 25/09/2008 1 1 1 04/11/2008 05/11/2008 06/11/2008 Brown Rocks Brown Rocks Brown Rocks Brown Rocks Brown Rocks 54.4539 -5.5937 22.1 Quadrats 54.4539 -5.5937 22.3 Quadrats 54.4539 -5.5937 21.7 Quadrats 54.4292 -5.6168 27 Exploratory (video) 54.4300 -5.6147 54.4292 -5.6168 22.4 Exploratory (video) 54.4292 -5.6168 19 Quadrats 54.4292 -5.6168 20 Quadrats 54.4292 -5.6168 21 Quadrats 54.4310 54.4292 -5.5976 -5.6335 15 22.5 17 25 Exploratory Exploratory Exploratory Exploratory 54.4539 -5.5937 28 DPV mapping 54.4536 -5.5962 22.6 DPV mapping 54.4555 -5.5964 226 13/01/2009 2 05/03/2009 2 06/03/2009 2 12/03/2009 2 13/03/2009 2 19/03/2009 2 20/03/2009 2 24/03/2009 2 South Hadd Rock (good condition) South Hadd Rock (good condition) South Hadd Rock (good condition) Hadd Rock (Buoyed line) Hadd Rock (Buoyed line) Hadd Rock (Buoyed line) Hadd Rock (Buoyed line) Hadd Rock (Buoyed line) 30/03/2009 2 30/03/2009 2 31/03/2009 2 31/03/2009 31/03/2009 01/04/2009 02/04/2009 2 2 2 2 Hadd Rock (Buoyed line) South Hadd Rock (poor site) South Hadd Rock (poor site) South Hadd Rock (poor site) Ringhaddy E. Brown Rock E. Brown Rock 20/04/2009 2 Brown rocks 21/04/2009 2 Brown rocks 21/04/2009 2 24/04/2009 1 Brown rocks East of Craigyouran East of Craigyouran East of Craigyouran East of Craigyouran 13/05/20009 1 Ringhaddy 1 Hadd Rock (Buoyed line) 1 23/04/2009 1 1 25/05/2009 54.4539 -5.5937 22.3 Quadrats 54.4539 -5.5937 22.3 Quadrats 54.4539 -5.5937 54.4545 -5.5943 Spat collectors Spat collectors Spat collectors 24 Spat collectors Retrieve spat collectors check for any remaining spat collectors 54.4534 -5.5914 20.1 Quadrats (N. poor site) 54.4534 -5.5914 24.8 Quadrats (N. poor site) 54.4534 54.4534 54.4292 54.4292 -5.5914 -5.5914 -5.6168 -5.6168 23.9 12 21 19 54.4421 -5.5826 36.3 Exploratory mapping 54.4422 -5.5804 36.3 Exploratory mapping 54.4426 -5.5833 Exploratory mapping 54.4428 -5.5826 54.4465 -5.6265 Exploratory mapping Collection of spat collectors Collect small M.modiolus. Collect M.modiolus shell Quadrats (N. poor site) spat collectors Quadrats (S.poor site) Quadrats (S.poor site) Quadrats (S. goood site) Quadrats (S. goood site) Quadrats (S. goood site) 227 25/05/2009 1 01/06/2009 1 1 03/06/2009 2 1 03/06/2009 Hadd Rock (Buoyed line) East of Sheila's island Selk Rock Selk Rock Ballyhom Bay Collect small M.modiolus. Collect M.modiolus shell 54.4949 -5.5598 Exploratory/mapping SMILE project diving 54.4178 54.4112 -5.6260 -5.6235 54.4198 54.4206 54.4179 54.4175 -5.6208 -5.6252 -5.6256 -5.6263 Exploratory/mapping Exploratory/mapping Exploratory/mapping Exploratory/mapping Exploratory/mapping Exploratory/mapping Exploratory/mapping Spat collectors(Pecten, Modiolus, empty Modiolus) Deployment of spat collector trays (live Mod, Pecten, empty Mod) Collection of 300 Modiolus 05/06/2009 1 1 1 1 08/06/2009 1 Brown Rocks 54.4295 -5.6243 10/06/2009 2 54.4518 -5.6024 18 15/06/2009 1 22/06/2009 1 Scott‟s Hole west of Brown Rocks Holm Bay/west of Limestone Pladdy 54.4175 -5.6263 16 23/06/2009 2 Holm Bay 54.9169 -5.6262 24/06/2009 2 1 1 54.4180 54.4167 54.4322 -5.6254 -5.6256 -5.6251 54.4172 -5.6024 30/06/2009 1 2 01/07/2009 1 3 06/07/2009 08/07/2009 10/07/2009 16/07/2008 1 1 1 1 1 2 Southern Experimental site Southern experimental site (holm Bay) Hadd Rock (SLECI quadrats) Chapel Island/ scallop collection site North of jackdaw island Kate‟s Pladdy Mc Loughlin‟s rock Limestone rock Spat collectors Collection of live Modiolus Collection of live Modiolus Deploy fragmentation sacks Scallop collection for AFBI 54.4113 -5.5824 54.3978 54.3952 54.3955 -5.6180 -5.6135 -5.6182 Exploratory dive Brown rocks 228 06/08/2009 07/08/2009 2 2 13/08/2009 2 24/08/2009 2 25/08/2009 2 Southern exp site(Holm Bay) Scott‟s Hole S.Hadd (good quad site) S.Hadd (poor quad site) Holm Bay(collection part II) Sack Experiment 54.4520 54.4169 -5.6020 -5.6247 16/09/2009 1 01/10/2009 2 05/10/2009 1 07/10/2009 1 08/10/2009 1 Holm Bay Scott‟s Hole (clumping experiment part (II) N.Long Sheelah N.Long Sheelah N.W Long Sheelah (T 14) heading south S.E Janes Rock (T16) heading south Slave Rock Transect 8 (in) N long Sheelah (t 11 to 12) East of Brown rocks East of sand rock Scott‟s Hole ( Sack exp (II) ) Scott‟s Hole ( Trays) Mooring inspection West of brown Rock Pladdy 12/10/2009 1 Browns rocks 54.4222 -5.6155 12/10/2009 1 54.4219 -5.6208 14/10/2009 4 Browns rocks Holm Bay Collection Site 54.4169 -5.6247 01/09/2009 2 1 09/09/2009 1 2 10/09/2009 2 15/09/2009 1 Collection for live Modiolus (experiment part (II)) Collection for live Modiolus (experiment part (II)) Mixed shell on sandy mud with Antedon Repeat SLECI transect Repeat SLECI transect 54.4499 -5.6026 54.4542 -5.5949 54.4552 -5.5944 54.4661 -5.5980 15.7 54.4459 -5.5993 21.2 54.4600 -5.5828 32.8 54.4503 -5.5974 16 54.4510 -5.5980 54.4510 -5.5980 54.4518 -5.6024 54.4297 -5.6240 20 16.2 Repeat SLECI transect Repeat SLECI transect Repeat SLECI transect Repeat SLECI transect Repeat SLECI transect Repeat SLECI transect Open and inspect sacks Spat collector retrieval C-mar mooring inspection 17.8 Spat collector retrieval Site selection for cultch deployment ( H. Edwards recommended site) site selection for cultch deployment ( H. Edwards recommended site) Collect modiolus for cultch experiment 229 23/11/2009 Scott‟s Hole 54.4497 -5.6035 19.5 54.3868 -5.5973 20 Collect modiolus shell for spat collectors Scallop collection for AFBI 30/11/2009 4 02/12/2009 3 04/12/2009 3 Long Sheelah Chapel Island/ scallop collection site Cultch deployment site 07/12/2009 3 Kates Pladdy 16/12/2009 2 Hadd rock 20/01/2010 2 Kates Pladdy 22/01/2010 2 Hadd Rock 54.4542 -5.5930 20.3 26/01/2010 3 Holm Bay 54.4169 -5.6247 17.3 27/01/2010 3 54.4169 -5.6247 17 02/02/2010 6 54.4213 -5.6203 19.7 Reposition cultch bags 03/02/2010 6 Holm Bay Cultch deployment site Cultch deployment site Deployment of additional cultch bags Scallop collection for AFBI Spat collector deployment Divers from Wales and IOM to see Strangford Lough Modiolus Spat collector deployment MMRG Spat collector deployment and modiolus collection Collect modiolus for cultch experiment 54.4213 -5.6203 17.8 10/02/2010 2 Black Rock 54.4297 -5.6240 22.2 54.4213 -5.6203 16.9 Reposition cultch bags Find trays and YSI Instrument - achieved Map out cultch site and attempt to reposition marked bags 54.4213 -5.6203 18.5 Reposition cultch bags 54.4213 -5.6203 23.6 54.4213 -5.6203 20 Reposition cultch bags Reposition cultch bags and relocate mooring block 54.4213 -5.6203 18.6 19/02/2010 4 22/02/2010 4 23/02/2010 4 24/02/2010 4 25/02/2010 3 Cultch deployment site Cultch deployment site Cultch deployment site Cultch deployment site Cultch deployment site 26/02/2010 2 Hadd Rock 24 54.4542 -5.5930 21 23.3 21 Scallop collection for AFBI Reposition cultch bags Search for spat collectors and collect modiolus for gonad samples 230 1 Holm Bay 54.4169 -5.6247 18.5 02/03/2010 1 Holm Bay 54.4169 -5.6247 14.1 03/03/2010 3 Holm Bay 54.4169 -5.6247 14 04/03/2010 2 54.4169 -5.6247 12.5 09/03/2010 3 54.4213 -5.6203 18.8 Flatten cultch plots 11/03/2010 2 54.4213 -5.6203 18.2 Flatten cultch plots 3 Holm Bay Cultch deployment site Cultch deployment site Cultch deployment site Search for spat collectors and collect modiolus for gonad samples Collect modiolus for cultch experiment Collect modiolus for cultch experiment Collect modiolus for cultch experiment 54.4213 -5.6203 20.4 1 Hadd Rock 54.4542 -5.5930 20.5 1 Holm Bay 54.4172 -5.6267 13.6 Cultch deployment site 54.4213 -5.6203 16.6 1 Hadd Rock 54.4542 -5.5930 17.6 1 Holm Bay 54.4172 -5.6267 12.2 54.4213 -5.6203 19.9 54.4213 -5.6203 19.6 54.4213 -5.6203 19.7 54.4213 -5.6203 20.3 Continue moving of modiolus onto plots Deploy flags, sediment traps, and complete modiolus movement on to plots. Video survey of plots. Finish flagging and translocation of modiolus on to plots 54.4280 -5.6174 26 SLECI repeat transect 54.4476 -5.6143 32 SLECI repeat transect 54.4641 -5.6138 34 SLECI repeat transect 12/03/2010 24/03/2010 25/03/2010 4 29/03/2010 3 12/04/2010 3 13/04/2010 2 1 14/04/2010 1 1 Cultch deployment site Cultch deployment site Cultch deployment site Cultch deployment site East of Black Rock transect 1 East of Black Rock transect 2 N. Green Island Passage Continue to arrange cultch plots Check new mooring block site Check new mooring block site Continue arranging cultch plots, light and temperature logger deployment Light and temperature logger deployment Light and temperature logger deployment Finish cultch plot arrangement, and begin to translocate modiolus 231 15/04/2010 1 54.4520 -5.5890 34.7 54.4213 -5.6203 17.7 26 SLECI repeat transect Deploy sediment traps, and baseline cultch survey. SLECI repeat transect 54.4336 -5.5912 30.8 SLECI repeat transect 54.4336 -5.5912 31 SLECI repeat transect 2 Hadd Rock Cultch deployment site Hadd Rock Round Island Pinnacle transect 1 Round Island Pinnacle transect 2 Cultch deployment site 19/04/2010 20/04/2010 2 2 54.4213 -5.6203 16.6 2 Hadd Rock 54.4542 -5.5930 24.5 2 Holm Bay 54.4172 -5.6267 15.8 1 Hadd Rock 54.4542 -5.5930 17.2 1 Holm Bay Cultch deployment site Holm Bay Mooring Block Cultch deployment site 54.4172 -5.6267 11.7 54.4213 -5.6203 26 54.4172 -5.6267 12.2 54.4213 -5.6203 17.8 1 1 21/04/2010 26/04/2010 05/05/2010 1 1 1 1 1 2 06/05/2010 1 2 14/05/2010 2 17/05/2010 1 18/05/2010 2 19/05/2010 2 20/05/2010 1 AFBI Buoy Hadd Rock Mooring Block N. Long Sheelah Round Island Mooring Block Round Island Mooring Block Cultch deployment site Brown Rocks Trays Brown Rocks Trays Brown Rocks Trays Brown Rocks Trays 9.9 54.4542 -5.5930 17 18.6 28.5 54.4344 -5.5871 30.6 54.4213 -5.6203 21.5 54.4297 -5.6240 20.5 54.4297 -5.6240 18.5 54.4297 -5.6240 18.8 54.4297 -5.6240 18.9 Cultch baseline survey - plot heights Collect Modiolus for hatchery broodstock Collect Modiolus for hatchery broodstock Retrieval of temperature and light loggers Retrieval of temperature and light loggers Retrieval of temperature and light loggers/ initial Deploy data loggers and spat collectors. Data logger deployment. Connect ADCP frame to AFBI Buoy Data logger deployment. Modiolus collection for size frequency and age analysis Data logger deployment. Mooring block inspection ROV at cultch site for fish monitoring. Find and photograph tray experiment Begin removal of trays Collect all materials from trays Complete removal of tray material. 232 1 Brown Rocks transect 1 Brown Rocks transect 2 Long Sheelah transect 1 Long Sheelah transect 2 AFBI Buoy South of Hadd Rock transect 1 South of Hadd rock transect 2 Between Long Sheelah and Hadd Rock Transect 1 Between Long Sheelah and Hadd Rock Transect 2 North of Round Island Pinnacle transect 1 North of Round Island Pinnacle transect 2 Sand Rock transect 1 Sand Rock transect 2 Round Island Pinnacle Mooring Block Cultch deployment site Holm Bay Mooring Block 2 Hadd Rock 54.4540 -5.5930 26.3 2 Holm Bay Green Island Passage to Great Minis transect1 Green Island Passage to Great Minis transect2 Scott‟s Hole Transect 1 54.4169 -5.6247 15.7 Sediment trap deployment Sediment trap deployment Collection dive and spat collector search Collection dive and spat collector search 54.4645 -5.6124 18 SLECI repeat transect 54.4638 -5.6062 16.5 SLECI repeat transect 54.4498 -5.6068 22.2 SLECI repeat transect 1 27/05/2010 1 1 28/05/2010 1 1 1 1 1 31/05/2010 1 1 1 1 02/06/2010 1 2 1 03/06/2010 04/06/2010 2 07/06/2010 2 08/06/2010 2 54.4287 -5.4477 36.7 SLECI repeat transect 54.4287 -5.6178 27.2 SLECI repeat transect 16.5 SLECI repeat transect 54.4498 -5.5981 16.5 9 SLECI repeat transect 54.4529 -5.5893 30.1 SLECI repeat transect 54.4511 -5.5889 27.8 SLECI repeat transect 54.4554 -5.5920 31.3 SLECI repeat transect 54.4538 -5.5923 31.7 SLECI repeat transect 54.4336 -5.5912 27.7 SLECI repeat transect 25 SLECI repeat transect 17.5 SLECI repeat transect 18 SLECI repeat transect Data logger and sediment trap deployment 54.4344 -5.5871 29.3 54.4213 -5.6203 17.2 54.4172 -5.6267 13.2 233 2 Scott‟s Hole transect 2 Round Island Pinnacle Mooring Block Long Sheelah transect 1 Long Sheelah transect 2 2 2 E. Black Rock Craigyouran 2 Craigyouran 2 11/06/2010 2 2 14/06/2010 17/06/2010 22/06/2010 2 24/06/2010 2 1 1 1 Scott‟s Hole Scott‟s Hole Trays Round Island Pinnacle Mooring Block 54.4502 -5.6079 20.5 54.4344 -5.5871 25 SLECI repeat transect Modiolus collection for size frequency and age analysis 54.4506 -5.5996 17.6 SLECI repeat transect 54.4497 -5.5998 18.6 54.4282 -5.6169 22.7 SLECI repeat transect Modiolus collection for size frequency and age analysis Too deep for planned collection 43.8 43 54.4518 -5.6024 20 Locate Scott‟s Hole Tray experiment 54.4518 -5.6024 20 Remove tray contents 54.4344 -5.5871 28 1 Craigyouran AFBI Buoy Hadd Rock Mooring Block Cultch deployment site Holm Bay Mooring Block Hadd Rock Mooring Block Cultch deployment site Holm Bay Mooring Block 1 Hadd Rock 20.5 20/07/2010 1 Holm Bay 17.9 23/07/2010 1 26 26/07/2010 2 27/07/2010 2 11/08/2010 2 Drop Off East Black Rock North Long Sheelah Round Island Pinnacle Mooring Block Deploy data loggers and sediment traps Deploy data loggers and sediment traps Gonad sample collections Gonad sample collections Video site, familiarisation dive 1 1 30/06/2010 1 1 2 16/07/2010 35.5 10 Sediment trap collection Modiolus collection for size frequency and age analysis ADCP collection Sediment trap collection Sediment trap and spat collector collection Sediment trap collection Deploy data loggers and sediment traps 54.4542 -5.5930 23.6 54.4213 -5.6203 19.5 54.4172 -5.6267 14 54.4542 -5.5930 17.8 54.4213 -5.6203 18.2 54.4172 -5.6267 13.4 54.4279 -5.6158 25 Quadrat total removal 54.4547 -5.5932 29.1 54.4344 -5.5871 33.3 Quadrat total removal Quadrat total removal/Search for Mooring Block 234 18/08/2010 1 1 1 08/09/2010 1 1 1 1 09/09/2010 1 1 22/09/2010 2 1 3 06/10/2010 1 1 20/10/2010 2 1 1 1 01/11/2010 1 2 07/12/2010 1 08/12/2010 2 09/12/2010 13/12/2010 2 2 Round Island Pinnacle Mooring Block Holm Bay Sacks Holm Bay Mooring Block Scott‟s Hole Sacks Scott‟s Hole Sacks Hadd Rock Collection Holm Bay Collection Site "Sacks" waypoint Holm Bay West of RIP way point Cultch deployment site Hadd Rock Cultch deployment site Holm Bay Mooring Block Round Island Pinnacle Mooring Block Cultch deployment site Hadd Rock Holm Bay Mooring Block Cultch deployment site Holm Bay Mooring Block Brown Rocks TRQ Long Sheelah TRQ Hadd Rock TRQ Round Island Pinnacle TRQ Round Island 54.4344 -5.5871 29 54.4172 -5.6024 14.8 Deploy data loggers Locate sacks unsuccessful 54.4172 -5.6267 14.8 Data logger retrieval 54.4499 -5.6026 30 Sack experiment 54.4499 -5.6026 14.8 54.4542 -5.5930 21.2 54.4169 -5.6247 14.6 Sack experiment Gonad sample collections Gonad sample collections 14.6 31.4 54.4213 54.4542 -5.6203 -5.5930 19.7 22.2 54.4213 -5.6203 20.9 54.4172 -5.6267 15 Video and photo samples, predator prey sample collection, general area mapping Secure ADCP from Cultch site 54.4344 -5.5871 32 ADCP collection 54.4213 54.4542 -5.6203 -5.5930 18 19 54.4172 -5.6267 15 54.4213 -5.6203 17.5 Collect data loggers 54.4172 -5.6267 13 Collect data loggers Total Removal Quadrats Total Removal Quadrats Total Removal Quadrats Total Removal Quadrats Total Removal 30 30 36 29.4 21.3 Cultch site survey and clean lines 235 14/12/2010 1 Hadd Rock Mooring Block 54.4542 -5.5930 20.1 1 1 Holm Bay Mooring Block Hadd Rock 54.4172 54.4542 -5.6267 -5.5930 13.1 17 Hadd Rock Mooring block Gonad sample collections and data logger retrieval 236 Appendix 3: Modiolus Researchers Group Meeting 19-20 January 2010 Program – January 19th : Location: Exploris education suite Time 9.30 10:00 Speaker Registration Bernard Picton 10:30 Joe Breen 10:40 11.00 11:20 Dai Roberts Break Anne M. Mahon 11:50 12: 30 Title M. modiolus communities in Strangford Lough, diving observations of changes since 1976. Background to creation of the Modiolus Restoration Research Project The Modiolus Restoration Research Project Current status of M. Modiolus in Strangford Lough Dave Smyth Restoration through cultch deployment and translocation Jose Fariñas Bill Sanderson Ivor Rees Experimental aquaculture of M. modiolus Variability in horse mussel reefs A description of an impoverished sand scoured Modiolus community Acoustic methods of monitoring in Modiolus beds 12.40 Charlie Lindenbaum 2:00 Bob Brown 2.20 2:40 Terry Holt Fiona Gell 3.00 3.20 4.00 Break Dan B Harris An overview of Modiolus research in Scottish waters Discussion Bob Brown Summary of talks/ discussion Dinner in the Portaferry Hotel 7.00 Geographical variation in the reproduction of M. modiolus The history of Modiolus around the Isle of Man Description of recently discovered Modiolus beds off the Isle of Man Chair of first session: Dai Roberts Chair of second session: Mark McCaughan Chair of third session: Graham Seymour Chair of fourth session: Bob Brown 237 Program – January 20th Time 9.30 10.00 11.30- 12.00 12.30 Activity Dive and ROV boats departing at 9.30 am from ferry slip Tour of marine lab, hatchery and video footage Boats return to Portaferry Tea, coffee and sandwiches in the seminar room 238 Appendix 4. List of epifauna and infauna recorded by the Modiolus Restoration Research Group during the survey period (2008-2010). For species codes see Howson & Picton (2000) Taxa Porifera Scypha ciliata Suberites carnosus Suberites ficus Cliona celata Halichondria panicea Mycale sp Mycale similaris Amphilectus fucorum Iophon hyndmani Myxilla incrustans Cnidaria Halecium halecinum Abietinaria abietina Hydrallmania falcata Sertularella gayi Sertularella polyzonias Sertullaria argentea Kirchenpaueria pinnata Nemertesia spp. Nemertesia antennina Nemertesia ramosa Plumularia setacea Aglaophenia pluma Obelia dichotoma Alcyonium digitatum Virgularia mirabilis Cerianthus lloydii Epizoanthus couchii Urticina eques Metridium senile Sagartia elegans Sagartiogeton laceratus Halcampa chrysanthellum Platyhelminthes Turbellaria sp. Species Directory Code C C C C C C C C C C 133 416 418 480 651 722 733 758 1052 1085 D D D D D D D D D D D D D D D D D D D D D D 392 409 424 429 430 434 455 462 463 466 469 481 519 597 617 632 649 683 710 713 721 758 F 2 239 Taxa Nemertea Tubulanus annulatus Cerebratulus fuscus Lineus longissimus Priapulida Priapulus caudatus Sipuncula Golfingia vulgaris Annelida: Polychaeta Adyte pellucida Harmothoe sp. Malmgrenia andreapolis Harmothoe impar Malmgrenia arenicolae Lepidonotus squamatus Polynoe scolopendrina Pholoe inornata Pholoe synophthalmica Sthenelais limicola Sthenelais boa Sthenelais zetlandica Eteone flava Eteone longa Eteone barbata Phyllodoce mucosa Pirakia punctifera Eumida bahusiensis Eumida sanguinea Paranaitis kosteriensis Glycera alba Glycera tridactyla Goniada maculata Sphaerodorum flavum Kefersteinia cirrata Nereimyra punctata Ophiodromus flexuosus Odontosyllis ctenosoma Odontosyllis gibba Nereis zonata Perinereis cultrifera Platynereis dumerilii Nephtys sp. Nephtys histricis Species Directory Code G G G 28 41 54 J 7 N 17 P P P P P P P P P P P P P P P P P P P P P P P P P P P P P P P P P P 32 50 51 65 67 82 84 92 94 106 107 111 117 118 126 145 157 164 167 176 256 265 271 291 305 311 313 386 388 478 480 484 494 500 240 Taxa Nephtys incisa Nephtys kersivalensis Lumbrineris latreilli Levinsenia gracilis Aonides paucibranchiata Polydora ciliata Prionospio fallax Spio spp. Spio filicornis Spiophanes bombyx Spiophanes kroyeri Aphelochaeta marioni Caulleriella zetlandica Chaetozone setosa Cirratulus sp Cirratulus cirratus Aphelochaeta filiformis Tharyx spp. Flabelligera affinis Pherusa plumosa Capitella capitata Mediomastus fragilis Notomastus latericeus Arenicola marina Euclymene sp. Euclymene oerstedii Ophelina acuminata Scalibregma celticum Scalibregma inflatum Myriochele heeri Owenia fusiformis Terebellida sp Lagis koreni Sabellaria alveolata Melinna palmata Ampharete sp. Ampharete lindstroemi Amphicteis gunneri Terebellides stroemi Terebellidae spp Eupolymnia nebulosa Eupolymnia nesidensis Pista cristata Species Directory Code P 501 P 502 P 582 P 693 P 723 P 752 P 765 P 787 P 790 P 794 P 796 P 824 P 831 P 834 P 835 P 836 P 837 P 847 P 881 P 885 P 907 P 919 P 921 P 931 P 961 P 964 P 1014 P 1026 P 1027 P 1096 P 1098 P 1099 P 1107 P 1116 P 1124 P 1133 P 1139 P 1142 P 1175 P 1179 P 1189 P 1190 P 1217 241 Taxa Polycirrus medusa Polycirrus plumosus Streblosoma intestinalis Branchiomma bombyx Euchone rubrocinta Euchone southernii Myxicola infundibulum Sabella pavonina Hydroides norvegica Pomatoceros sp Pomatoceros lamarckii Pomatoceros triqueter Serpula vermicularis Annelida: Oligochaeta Tubificoides spp. Chelicerata Pycnogonida Crustacea: Cirripedia Semibalanus balanoides Balanus spp. Balanus balanus Crustacea: Leptostraca Nebalia bipes Crustacea: Mysidacea Mysis sp. Crustacea: Amphipoda Lysianassa ceratina Tryphosella sarsi Atylus sp. Dexamine spinosa Ampelisca spinipes Ampelisca typica Jassa falcata Maera othonis Corophium sp. Corophium sextonae Crustacea: Decapoda Apseudes talpa Palaemon elegans Palaemon serratus Thoralus cranchii Pandalus montagui Nephrops norvegicus Species Directory Code P 1242 P 1244 P 1252 P 1263 P 1280 P 1281 P 1300 P 1320 P 1334 P 1339 P 1340 P 1341 P 1343 P 1487 Q 2 R R R 70 74 76 S 6 S 31 S S S S S S S S S S 303 344 409 415 438 442 509 519 605 615 S S S S S S 1177 1317 1319 1360 1377 1402 242 Taxa Upogebia pusilla Anapagurus sp. Pagurus bernhardus Galathea intermedia Galathea strigosa Munida rugosa Pisidia longicornis Hyas araneus Inachus dorsettensis Inachus phalangium Macropodia rostrata Eurynome spinosa Cancer pagurus Liocarcinus spp. Liocarcinus depurator Liocarcinus pusillus Necora puber Carcinus maenas Pinnotheres pisum Mollusca: Polyplacophora Leptochiton asellus Hanleya hanleyi Callochiton achatinus Acanthochitona crinitus Mollusca: Gastropoda Diodora graeca Gibbula spp. Gibbula cineraria Calliostoma zizyphinum Turritella communis Calyptraea chinensis Capulus ungaricus Trivia monacha Boreotrophon truncatus Buccinum undatum Hinia reticulata Hinia incrassata Mollusca: Nudibranchia Tritonia hombergii Onchidoris sp. Jorunna tomentosa Mollusca: Pelecypoda Nucula nucleus Species Directory Code S 1420 S 1446 S 1457 S 1472 S 1476 S 1478 S 1482 S 1518 S 1526 S 1528 S 1532 S 1537 S 1566 S 1577 S 1580 S 1584 S 1589 S 1594 S 1638 W W W W 53 65 75 85 W W W W W W W W W W W W 116 158 163 182 270 436 443 461 680 708 745 747 W W W 1250 1320 1386 W 1570 243 Taxa Species Directory Code Nucula sulcata W 1571 Mytilus edulis W 1695 Modiolus modiolus W 1702 Modiolarca tumida W 1718 Ostrea edulis W 1758 Pecten maximus W 1771 Aequipecten opercularis W 1773 Chlamys varia W 1779 Palliolum tigerinum W 1786 Anomia ephippium W 1807 Pododesmus patelliformis W 1814 Loripes lucinalis W 1824 Myrtea spinifera W 1827 Lucinoma borealis W 1829 Thyasira flexuosa W 1837 Mysella bidentata W 1906 Astarte sulcata W 1925 Gari fervensis W 2051 Abra alba W 2059 Venus verrucosa W 2089 Timoclea ovata W 2104 Venerupis spp. W 2110 Tapes spp. W 2111 Tapes rhomboides W 2113 Venerupis senegalensis W 2124 Dosinia lupinus W 2128 Mya sp. W 2145 Mya truncata W 2147 Corbula gibba W 2157 Hiatella arctica W 2166 Bryozoa Crisia spp. Y 13 Crisia denticulata Y 16 Crisia eburnea Y 17 Alcyonidium diaphanum Y 76 Membranipora membranacea Y 170 Electra pilosa Y 178 Flustra foliacea Y 187 Bugula flabellata Y 243 Scrupocellaria reptans Y 276 Scrupocellaria scruposa Y 279 Cellaria spp. Y 299 Cellaria fistulosa Y 300 244 Taxa Cellepora pumicosa Echinodermata Antedon bifida Crossaster paposus Henricia spp. Henricia oculata Asterias rubens Leptasterias muelleri Marthasterias glacialis Ophiothrix fragilis Ophiocomina nigra Amphiura chiajei Amphiura filiformis Amphipholis squamata Ophiura sp. Ophiura albida Ophiura ophiura Psammechinus miliaris Echinus esculentus Thyone spp. Thyone fusus Thyone roscovita Ocnus brunneus Ocnus lacteus Leptopentacta elongata Thyonidium drummondi Tunicata Tunicata indet. Clavelina lepadiformis Ciona intestinalis Diazona violacea Corella paralelogramma Ascidiella aspersa Ascidiella scabra Ascidia mentula Styela gelatinosa Polycarpa scuba Pyura microcosmus Pyura tesselata Pisces Lepadogaster candolii Trisopterus minutus Eutrigla gurnardus Species Directory Code Y 495 ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB ZB 10 75 82 83 100 102 104 124 128 152 154 161 166 168 170 193 198 261 262 264 274 275 280 281 ZD ZD ZD ZD ZD ZD ZD ZD ZD ZD ZD ZD 1 7 71 74 81 84 85 89 106 116 139 141 ZG ZG ZG 88 144 265 245 Taxa Lipophrys pholis Pholis gunnellus Callionymus lyra Gobius niger Pomatoschistus minutus Pomatoschistus pictus Species Directory Code ZG 412 ZG 440 ZG 452 ZG 467 ZG 479 ZG 481 246