SCH 3U1 COMBUSTION REACTIONS Combustion reactions are important to society because of
advertisement

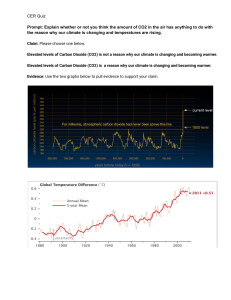
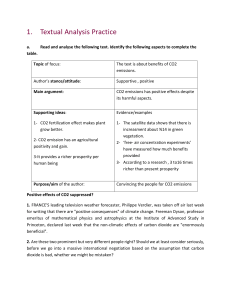
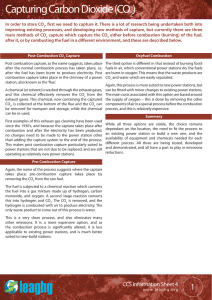
SCH 3U1 COMBUSTION REACTIONS Combustion reactions are important to society because of the heat they produce. They are also potentially harmful to us due to the pollution they cause. Carbon dioxide, a greenhouse gas is a pollutant because the inhabitants of the earth produce more CO2 than the earth can photosynthesize. This means that the levels of CO2 in the atmosphere increase slightly. C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g) H = -2041 kJ/mol The heat term at the end of the equation shows that heat is released. This is called an exothermic reaction. From the point of view of potential (bond) energy this reaction is tells us the bonds of propane and oxygen are at a higher potential than water and carbon dioxide. The reaction involves the following bonds: C-C, C-H + O=O C=O + H-O The total energy of the products equals the energy of the reactants. If the reaction generates heat (kinetic) energy then there must be a decrease in potential energy of the products. PE Eactivation H = -ve time Potential energy in chemistry refers to bond energy.