SCH 3U Three ways of writing chemical equations: 1. Word equations
advertisement

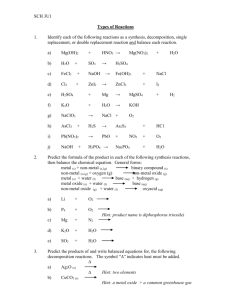
SCH 3U Three ways 1. 2. 3. - Chemical Reactions of writing chemical equations: Word equations Skeleton equations Balanced chemical equations Evidence of a Chemical Reaction change in colour - formation of a gas change in heat/light - formation of a precepitate change in odour Law of Conservation of Mass In any chemical reaction, the mass of the products is always equal to the mass of the reactants. Matter can neither be created nor destroyed. Rules for Balancing Chemical Equations 1. Balance atoms/polyatomic ions left to right. Leave oxygen and hydrogen until the end. 2. Balance hydrogen and oxygen leaving the one that appears in the most compounds last. Classification of Chemical Reactions 1. Synthesis Reactions E + E C or C + C C or C+E→C element + oxygen oxide eg. C(s) + O2(g) ____(g) metal + non-metal binary compound (salt) __ Zn(s) + S8(s) __ ____(s) 2. Decomposition CE+E or C C + C or C→C+E Eg. H2CO3 H2O + CO2 or __ NaCl ______________ 3. Combustion Rapid reaction of a substance with oxygen. Substances containing carbon and hydrogen (fuels) form carbon dioxide and water during combustion: C3H8 (g) + O2 (g) CO2 (g) + H2O(g) Incomplete combustion of fuels results in production of carbon monoxide (poisonous gas) and/or soot (C). C2H4 (g) + O2 (g) CO (g) + H2O(g) 4. Single Displacement A + BC AC + B or AB + C AC + B Metal element replaces metal, e.g. Cu + 2AgNO3 ____________ Non-metal element replaces non-metal, eg. Cl2 + 2KBr ________ 1+ Cation: positive ion, usually a metal or H . Anion: negative ion, usually a non-metal. The activity series for metals determines whether a reaction will occur. Li > K > Ca > Na > Mg…Au. There is a displacement if the metal is higher than the metal ion on the activity series: Fe + Hg(NO3)2 ____________ – Fe higher than Hg2+ Zn + MgCl2 ______________ – Zn lower than Mg2+ There is an activity series for halogens as well: F > Cl > Br > I 5. Double Displacement Reactions C+C→C+C Will form: AB + CD → AD + CB a precipitate (solid that is formed) a gas (decomposition of a product) salt and water (neutralization reaction) If both compounds remain in solution than there has been no reaction. NaCl(aq) + AgNO3(aq) → _______________________ 2 HCl(aq) + MgCO3(s) → _______________________ H2CO3(aq) → H2O(l) + CO2(g) KOH(aq) + HNO3(aq) → ________________________