3.3 Review & Practice Name________________________________ Period_______Date_____________________
advertisement

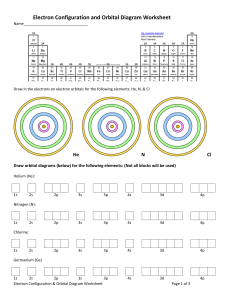
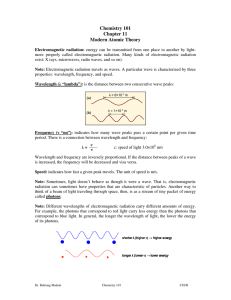
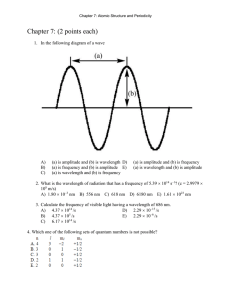
3.3 Review & Practice Name________________________________ Electron Configuration & Orbital Box Diagrams Period_______Date_____________________ Part I: True and False - If the statement is true write “true.” If it is false, change the underline word or words to make it correct. _______________ 1. The Pauli Exclusion Principle states that an orbital can hold a maximum of two electrons. _______________ 2. The sum of the superscripts in an electron configuration represents the total number of neutrons in the atom or ion. _______________ 3. The Aufbau Principle states that electrons are added one at a time to the highest energy orbitals available until all the electrons of the atom have been accounted for. _______________ 4. An orbital box diagram uses arrows to represent the spin of the electrons _______________ 5. The ground state is the least stable energy state of an atom. _______________ 6. According to Hund’s Rule, electrons occupy equal energy orbitals so that a maximum number of unpaired electrons result. Part II: Completion – Identify the elements (atoms) that have the following electron configurations. Write the chemical symbol for each element in the space provided. _________ 7. 1s22s22p3 ________ 12. 1s22s22p63s23p64s23d104p5 _________ 8. 1s22s22p63s23p2 ________ 13. 1s22s22p6 _________ 9. 1s22s22p63s23p64s23d10 ________ 14. 1s22s22p63s23p64s23d4 _________ 10. 1s22s22p63s23p64s2 ________ 15. 1s22s22p63s23p64s23d104p65s24d10 _________ 11. 1s22s22p63s23p64s23d104p65s24d105p66s24f145d6 Part III: Short Answer 16. Explain the Aufbau Principle, Pauli Exclusion Principle and Hund’s Rule when predicting an electron configuration. _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ Part IV: Write the Electron Configuration on the first line and Orbital Box Diagram on the second line. x 2 17.magnesium 2 6 2 1s 2s 2p 3s ______ y z 1s 2s 2p 3s 18. oxygen _____________________________ ____________________________________ 19. aluminum _____________________________ ____________________________________ 20. argon _____________________________ ____________________________________ 21. scandium ___________________________________________________________________ ___________________________________________________________________ 22. phosphorus ___________________________________________________________________ ___________________________________________________________________ 23. potassium ___________________________________________________________________ ___________________________________________________________________ Part V: Write the electron configuration for each of the following elements and then write the number of unpaired electrons each atom has. Write the electron-dot diagram around each element’s symbol. 24. Na _________________________________________________________________________ 25. As _________________________________________________________________________ 26. Ir _________________________________________________________________________ 27. Se _________________________________________________________________________ 28. Bi _________________________________________________________________________ 29. Hg _________________________________________________________________________