Characteristic Physical Properties: Density & Boiling Point
advertisement

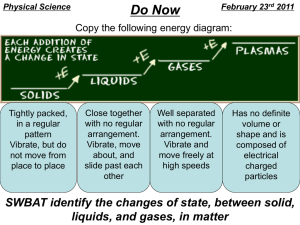
5.6: Characteristic Physical Properties pg. 192 Key Concepts: 1. Physical properties are characteristics that can be determined without changing the composition of the substance. 3. Pure substances have characteristic physical properties. 4. Water has unusual characteristic physical properties. Characteristic Physical Property: a physical property that is unique to a substance and that can be used to identify the substance. - Understanding unique properties and characteristics of pure substances can be used to determine the substance. - There are certain properties unique to pure substances, these are known as characteristic properties and are highly specific to the substance. - There are three characteristic physical properties that are important: - Density - Freezing/melting point - Boiling point Density Density: a measure of how much mass is contained in a given unit of volume of a substance, calculated by dividing the mass of a sample by its volume. - Density is the ration between mass to the volume of a substance. It is measure in g/cm3 for a solid and g/ml for a liquid. - The density of a substance is related to the mass or the particles of which the substance is composed, even though it can be in the same size container similar to another substance. Figure 3: A greater mass of the same substance packed into a smaller volume has a greater density. - Gases density can be influenced by temperature and pressure. - Gases can be packed close together but when heated the gas particles move further apart and therefore become less dense. - An increase in pressure will make the gas dense, as particles are compacted closer together. - In liquids and solids, changes of temperature and pressure have little impact on density. Table 1: Densities of Common Metals (at room Temperature and Atmospheric Pressure) Metal Aluminum Zinc Iron Copper Silver Lead Mercury Gold Density (g/cm3) 2.70 7.13 7.87 8.96 10.49 11.36 13.55 19.32 Sample Problem: Using problem solving format (GRASP) Calculate the density of a metal sample that is 18.00 cm long, 9.12 cm wide, and 4.45 cm high and that has a mass of 14.25 kg. What is the identity of the metal? Given: Required: Analysis: Solution: Statement: equations units of measure Freezing Point, Melting Point, and Boiling Point Freezing Point: the temperature at which a substance changes state from a liquid to a solid, melting point and freezing point are the same temperature for a substance. Melting Point: the temperature at which a substance changes state from solid to a liquid. Boiling Point: the temperature at which a substance changes state rapidly from a liquid to a gas. - 0oC is a very significant temperature. Water freezes or melts at 0oC. - Different substance freeze or melt at different temperatures. - This is characteristic physical property, specific to each pure substance. - 100oC is the temperature that pure water boils and changes form a liquid to a gas. - The composition of a substance determines the temperature a substances can change states. Application of Melting Point - Knowing the melting point of certain metals is important for determining function of the metal. Light bulb – Tungsten filament – Heat and light produce by electricity. The filament can not melt when heated. - Metal with low melting point are very useful. Mercury is used for thermostats, it can conduct electricity and still is a liquid at room temperature. Salt and Ice - Slat melts ice, even though water freezes at 0oC. If there are impurities in the water, it will interfere with the freezing of water. - Salt is an impurity, it lowers the freezing point of water, and therefore it doesn’t freeze at 0oC. - 20% salt solution will now freeze at -16oC. The Unusual Behaviour of Water - Water is odourless, colourless, and tasteless. It freezes at 0oc and Boils at 100oC. - Water as a solid is less dense then water as a liquid, therefore it floats in water. - 4oC is the transition point. Water at 4oC is dense and sinks to the bottom, but below 4oC the water becomes less dense floats to the surface as it solidifies. Figure 10: Ice is less dense than water because the particles in the ice take up a larger volume than the same number of particles in liquid water. The mass of the particles remain the same. Evidence of Learning: Students can … - describe characteristic physical properties of pure substances and give examples. - calculate the density of a given substance. - describe the freezing point, melting point, and boiling point of pure substances. - discuss water’s unusual behaviour and its importance for living organisms. Check Your Learning: Questions 1 – 12, page 198 Summary: - A characteristic physical property is a physical property that is unique to a pure substance and that can be used to identify the substance. - Density is a measure of how much mass is contained in a given unit of volume of a substance. It is calculated by dividing the mass of a sample by its volume. - Melting point is the temperature at which a substance changes state from a solid to a liquid. - Freezing point is the temperature at which a substance changes state from liquid to a solid. Melting point and freezing point are the same temperature for a substance. - Boiling Point is the temperature at which a substance changes state rapidly from a liquid to a gas. - Unlike other substances, water that is close to its melting point is less dense in the solid state than in the liquid state. Therefore, ice floats on water.