Optical Tomographic Imaging of Small Animals Overview • Introduction
advertisement

Overview
Optical Tomographic Imaging
of Small Animals
• Introduction
X-Ray Tomography vs Optical Tomography
• Model-based iterative image reconstruction
Basic concepts and mathematical background
• Instrumentation
General optical imaging modalities
Dynamic optical tomography system
Andreas H. Hielscher, Ph.D.
• Applications
Brain Imaging
Tumor Imaging
Fluorescence Imaging
Columbia University, New York City
Dept. of Biomedical Engineering
Dept. of Radiology
Overview
X-Ray Imaging
Uses X-rays to generate shadowgrams M(ϕ,ξ).
• Introduction
Basic concepts and mathematical background
• Instrumentation
General optical imaging modalities
Dynamic optical tomography system
• Applications
Brain Imaging
Tumor Imaging
Fluorescence Imaging
(measurable
attenuation)
unknown
absorption cross-section
A(x,y)
X-ray source
• Model-based iterative image reconstruction
M(ϕ,ξ)
X-Ray Tomography vs Optical Tomography
electromagnetic
wave λ~10-10 m
energy~104eV
Energy propagates on
straight lines through medium
1
M(ϕ,ξ)
X-ray source
X-Ray Tomography
X-Ray Shadowgram
X-Ray Tomography
X-Ray Tomography
Xra
y
so
ur
ce
M
(ϕ
,ξ)
2
Xra
y
so
ur
ce
M
(ϕ
,ξ
)
X-Ray Tomography
2D Scan of Head
unknown
absorption cross-section
M(ϕ,ξ)
X-ray source
A(x,y)
=>Simple image reconstruction scheme:
backprojection of M on lines of transmission.
(Inverse Radon Transform)
Optical Imaging
Optical Shadowgram
Uses near-infrared light (700< λ<900nm)
A(x,y)
{unknown
absorption
&
scattering
profile}
light
source
EM - wave
λ ~ 800•10-9m
energy ~ 1 eV
Energy does not propagate on straight line between
source and detector (light is strongly scattered)
3
Optical Tomography
light
source
Optical Tomography
light
source
Optical Tomography
Optical Tomography
light
source
light
source
4
Overview
Optical Imaging
Uses near-infrared light (700< λ<900nm)
• Introduction
X-Ray Tomography vs Optical Tomography
A(x,y)
{unknown
absorption
&
scattering
profile}
• Model-based iterative image reconstruction
light
source
EM - wave
λ ~ 800•10-9m
energy ~ 1 eV
Basic concepts and mathematical background
• Instrumentation
General optical imaging modalities
Dynamic optical tomography system
• Applications
Brain Imaging
Tumor Imaging
Fluorescence Imaging
How to reconstruct cross-sectional images A(x,y)
from measurement on surface?
(Inverse Problem)
initial
guess
D=
1 cm2n
s
Forward Model I
detectors
?
Theory:
sources
Experiment
detectors
sources
Model-Based Iterative Image Reconstruction
Forward Model, F (
)
depends on NxN unkowns
measured
detector readings I M,i
predicted
detector reading I P,i(
3D-Time-Resolved Diffusion Equation
∂U = ∂ D ∂U + ∂ D ∂U + ∂ D ∂U - cµaU + S
∂z
∂x ∂y ∂y ∂z
∂t ∂x
)
with
c := speed of light in medium, S = Source,
and diffusion coefficient :
D = c ( 3 [ µa + µs' ] )
with µ a = absorption coefficient and
µ s' = reduced scattering coefficient .
5
Diffusion vs Transport Model
Limits of Diffusion Model
laser beam
ring filled
with water
∂U = ∇c/(3µ +3µ ') ∇U - cµaU + S
a
s
∂t
discretize into N spacial variables
leads to N finite-difference equations
milk
∫
discretization into N spacial and A angular variables
leads to N x A coupled finite-difference equations
1.5
Diffusion
1
Experiments
0.5
0
5
10
15
1
Experiments
0.8
0.6
0.4
Transport
0.2
0
slower by factor ~A
1.2
20
25
30
35
40
Transport
0
5
10
15
y [mm]
Forward Model applied to
Mouse Head
20
x [mm]
25
30
35
40
Model-Based Iterative Image Reconstruction
sources
Experiment
~ 1 cm
?
Theory:
initial
guess
D=
1 cm2n
s
detectors
4π
µs' = (1-g) µs
1.4
sources
4π
and
Diffusion
1.6
2
detectors
∫
with U = Ψ(Ω') dΩ'
1.8
2.5
Intensity [au]
equation of radiative transport
∂Ψ/c∂t = S - Ω ∇Ψ - (µa + µs)Ψ + Ψ(Ω') p(Ω∗Ω') dΩ'
Intensity [au]
approximation
diffusion equation
Forward Model, F (
)
depends on NxN unkowns
log
(Fluence
[Wcm -2])
measured
detector readings I M,i
predicted
detector reading I P,i(
)
source
µa=0.1 cm -1 , µs =10 cm -1 ; 14781 nodes, 24 ordinates
6
?
Theory:
new
guess
Forward Model, F (
)
e.g. transport equation
predicted
detector reading I P,i(
measured
detector readings I M,i
Analysis Scheme
Φ ≈ { I M,i - I P,i(
Σ
)
Forward Model, F (
)
e.g. transport equation
Analysis Scheme
Φ ≈ { I M,i - I P,i(
)}2
i
)
Error Value Φ (
no
Model-Based Iterative Image Reconstruction
Experiment
sources
new
guess
?
Theory:
new
guess
Forward Model, F (
)
e.g. transport equation
measured
detector readings I M,i
predicted
detector reading I P,i(
Analysis Scheme
Φ ≈ { I M,i - I P,i(
Σ
)}2
i
Updating Scheme
Model-Based Iterative Image Reconstruction
sources
Theory:
detectors
sources
Experiment
no
Φ<ε
detectors
Φ<ε
)
)
sources
Error Value Φ (
Forward Model, F (
)
e.g. transport equation
measured
detector readings I M,i
predicted
detector reading I P,i(
Analysis Scheme
Φ ≈ { I M,i - I P,i(
Σ
)
)}2
i
Error Value Φ (
yes
)
)}2
Σ
(This is just one number!)
?
predicted
detector reading I P,i(
measured
detector readings I M,i
i
yes
sources
Experiment
detectors
D=
1 cm2n
s
sources
initial
guess
Model-Based Iterative Image Reconstruction
detectors
?
Theory:
detectors
sources
Experiment
sources
Model-Based Iterative Image Reconstruction
Φ<ε
)
no
Error Value Φ (
Φ<ε
)
no
Updating Scheme
7
Experiment
?
Theory:
detectors
sources
?
new
guess
Model-Based Iterative Image Reconstruction
sources
Theory:
detectors
sources
Experiment
new
guess
Forward Model, F (
)
e.g. transport equation
measured
detector readings I M,i
predicted
detector reading I P,i(
Analysis Scheme
Φ ≈ { I M,i - I P,i(
Σ
Forward Model, F (
)
e.g. transport equation
measured
detector readings I M,i
)
)}2
Σ
)
)}2
i
Error Value Φ (
yes
predicted
detector reading I P,i(
Analysis Scheme
Φ ≈ { I M,i - I P,i(
i
final
sources
Model-Based Iterative Image Reconstruction
)
Error Value Φ (
final
yes
Φ<ε
Iteration Example
Φ<ε
)
no
Updating Scheme
Iterative Reconstruction
Initial Guess:
D = 1.0 cm 2ns -1
Detector Source
2nd Iteration
8th Iteration
D [cm/ns 2]
D [cm/ns 2]
8 cm
Intensity
0
predictions
Time Steps
(Δt = .05 ns)
50
7
0
measurements
7
7
predictions
0
Time Steps
50
homogeneous
initial guess
(D = 1 cm 2ns-1)
0.5
0.5
measurements
24th Iteration
1.5
1.5
0
0
Time Steps
50
0
0
Time Steps
50
iteratively change properties of medium
until measurements and predictions agree
4 cm
8
Image Reconstruction
as an Optimization Problem
Data Analysis Scheme
Find image for which error value is smallest !
objective
error
function
Φ(D,µa)
Measurement Data Y Predicted data U
(Ysdt - Usdt (µa,D))2
Φ(µa,D) =
2σ2sdt
s d t
Contour plot of Φ(D,µa)
ΣΣΣ
Objective
Function
µa
D
Gradient Path
Conjugate Gradient
Path
=
χ2 Error Function
Goal : Find minimum of Φ(µa,D)
Employ minimization technique
that uses information about gradient
dΦ(µa,D) .
d(µa,D)
each image = 40x40 unknowns
Gradient Calculation
Divided Difference
Gradient Calculation
Divided Difference
1 variable: 2 forward calculations
needed to get gradient
1 variable: 2 forward calculations
needed to get gradient
∂f(ζx) f(ζ2)- f(ζ1)
∂ζ = ζ2 - ζ1
∂f(ζx) f(ζ2)- f(ζ1)
∂ζ = ζ2 - ζ1
f(ζ1)
f(ζx)
f(ζ2)
ζ1 ζx ζ2
Therefore,
For problem with N unknowns
one needs 2N forward
calculations to find gradient.
f(ζ1)
f(ζx)
f(ζ2)
ζ1 ζx ζ2
Therefore,
For problem with N unknowns
one needs 2N forward
calculations to find gradient.
Adjoint Differentiation
The evaluation of a gradient
requires never more than
five times the effort of
one forward calculation!
A. Griewank, “On Automatic Differentiation,” in
Mathematical Programming, M. Iri, K. Tanabe, eds.,
Kluwer Academic Publishers, 1989, pp.83-107.
Therefore,
adjoint differentiation method is
2N/5 times faster than
”traditional” divided difference
scheme!
9
For more details see:
Overview
G. Abdoulaev, K. Ren, A.H. Hielscher, "Optical tomography as a constrained optimization
problem,” accepted for publication in Inverse Problems.
K. Ren, G. Abdoulaev, G. Bal, A.H. Hielscher, "Frequency-domain optical tomography based
on the equation of radiative transfer,” accepted for publication in SIAM Journal of Scientific
Computing.
K. Ren, G. Abdoulaev, G. Bal, A.H. Hielscher, "An algorithm for solving the equation of
radiative transfer in the frequency domain," Optics Letters 29(6), pp. 578-580 (2004).
G. Abdoulaev and A.H. Hielscher, "Three-dimensional optical tomography with the equation of
radiative transfer," Journal of Electronic Imaging 12(4), pp. 594-60 (2003).
A.H. Hielscher, A.D. Klose, U. Netz, J. Beuthan, "Optical tomography using the timeindependent equation of radiative transfer. Part 1: Forward model," Journal of Quantitative
Spectroscopy and Radiative Transfer, Vol 72/5, pp. 691-713, 2002.
A.D. Klose, A.H. Hielscher, "Optical tomography using the time-independent equation of
radiative transfer. Part 2: Inverse model," Journal of Quantitative Spectroscopy and
Radiative Transfer, Vol 72/5, pp. 715-732, 2002.
A.D. Klose and A.H. Hielscher, "Iterative reconstruction scheme for optical tomo-graphy based
on the equation of radiative transfer," Medical Physics, vol. 26, no. 8, pp. 1698-1707,
1999.
A.H. Hielscher, A.D. Klose, K.M. Hanson, "Gradient-based iterative image recon-struction
scheme for time-resolved optical tomography," IEEE Transactions on Medical Imaging 18,
pp. 262-271, 1999.
www.bme.columbia.edu/biophotonics
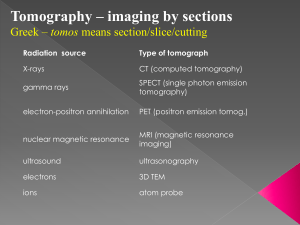
Optical Imaging Modalities
STEADYSTATE
DOMAIN
100k
X-ray vs optical tomography
• Model-based iterative image reconstruction
Basic concepts and mathematical background
• Instrumentation
General optical imaging modalities
Dynamic optical tomography system
• Applications
Brain Imaging
Tumor Imaging
Fluorescence Imaging
Frequency vs Steady-State Domain
1 image
/min
data acquisition rate
information content
FREQUENCY
DOMAIN
complexity/price of system
1M
TIME
DOMAIN
• Introduction
10 images
/sec
target
steady-state
frequency
domain
domain
reconstruction reconstruction
(ω = 0)
(ω = 600 MHz)
absorption
coefficient
µa
scattering
coefficient
µs‘
10
Instrument Diagram
Overview
optical fibers
• Introduction
detector
channels
X-ray vs optical tomography
• Model-based iterative image reconstruction
Basic concepts and mathematical background
• Instrumentation
rotating
mirror
coupler
tissue
SC SC SC SC
PC
DAQ
General optical imaging modalities
Dynamic optical tomography system
• Applications
LD 1
Brain Imaging
Tumor Imaging
Fluorescence Imaging
laser diodes
LD 2
LDD 1
LDD 2
Dynamic Optical Tomography System
(DYNOT)
PS 1
lock-in reference
PS 2
Dynamic Optical Tomography System
(details)
Arm
Detector Unit
Iris & Folding
Hemisphere
Timing Board
User Interface
& Software
Fiber Optics
Opto-deMUX
Laser Diodes & Driver
Student
Up to 10 full tomographic images per second!
11
Detector and Timing Boards
Detector modules
(lock-in detection scheme,
individual gain settings
2 amplification stages)
Interfacing Board
Timing Board
Back plane
Dynamic Optical Tomography System
(DYNOT)
From power supply
To DAQ board
Dynamic Range of Measurement
Dynamic Range of Measurement
~ 10-1 •0.01 W
0.1 W
~ 10-3 •0.1 W
0.01 W
~10-1 • 0.1 W
5 cm
5 cm
~ 10-5 •0.1 W
~ 10-5 •0.1 W
~10-3 •0.1 W
12
Dynamic Range of Measurement
Dynamic Range of Detectors
10
3 amplification stages to bring signal within 0.5 - 5 V
1
× 106
5 cm
10-1
× 103
Signal [ V ]
~10-5 •0.1 W
10-3
10-4
10-5
10-6
10-7
10-8
0.1 W
~ 10-3 •0.1 W
10-2
10-9
10-9 10-8 10-7 10-6 10-5 10-4 10-3 10-2 10-1
1
Nominal OD value
Timing Scheme
6 msec 6 msec
move mirror
to new fiber,
switch gains
target
illumination
(1 source)
Lock In
S/H
32
detectors
in parallel
DAQ
TASK
Src.1
Src. Pos.1
SETTL. TIME
SAMPLE
Src. 2
Src. Pos. 2
SETTL. TIME
Src. Pos. 3
HOLD
DATA
READ
SAMPLE
Src. 3
SETTL. TIME
HOLD
DATA
READ
SAMPLE
HOLD
TIME
Performance Overview
DATA
READ
Parameter
Value
Modulation frequency
5-10 kHz
Data acquisition rate
~150 Hz
Settling time
1-2 ms
Noise equivalent power
10 pW (rms)
Dynamic range
1:109 (180 dB)
Long term bias drifts
~1% over 30 min
Background light reject
~100 dB
13
For more details see:
A.H. Hielscher, A.Y. Bluestone, G.S.Abdoulaev, A.D. Klose, J. Lasker, M.
Stewart, U. Netz, J. Beuthan, "Near-infrared diffuse optical tomography,"
Disease Markers 18(5-6), pp. 313-337 (2002).
C.H. Schmitz, M. Löcker, J.M. Lasker, A.H. Hielscher, R.L. Barbour,
"Instrumentation for fast functional optical tomography," Rev. of
Scientific Instrumentation 73(2), pp. 429-439 (2002).
C.H. Schmitz, Y. Pei, H.L. Graber, J.M. Lasker, A.H. Hielscher, R.L.
Barbour, "Instrumentation for real-time dynamic optical tomography," in
Photon Migration, Optical Coherence Tomography, and Microscopy, S.
Andersson-Engels, M.F. Kaschke, eds., SPIE-The International Society
for Optical Engineering, Proc. 4431, pp. 282-291, 2001.
Overview
• Introduction
X-ray vs optical tomography
• Model-based iterative image reconstruction
Basic concepts and mathematical background
• Instrumentation
General optical imaging modalities
Dynamic optical tomography system
• Applications
Brain Imaging
Tumor Imaging
Fluorescence Imaging
www.bme.columbia.edu/biophotonics
Animal Model
Probe Geometry
Forehead shaven
325 gm
Sprague Dawley Rats
Animal’s head fixed in place using stereotaxic
5.0
mm
λ
4 sources
12 detectors
1.5
1.5
1.5
BP
Regulate inspired
[O2 ] and [CO 2 ]
Blood Pressure and
derived respiratory
rate via
Femoral catheter
1.5
Ant.
Ventilated at:
40-60 breaths/min
1-1.5 cc/breath
Optical probe with fixed geometry positioned in line with
lambda (λ) suture line, optodes begin 2 mm anterior to λ.
1.5
Anesthesia:
Urethane
administered i.p.
14
Probe Location
Carotid Occlusion
Dorsal view
posterior
λ
S1
S2
D9
D1
D5
D7
D6
D8
D4
D12
S3
S4
β
animal’s right
animal’s left
anterior
Carotid Occlusion
46.
35.
Hb [µM]
right occlusion
24.
13.
2.0
-3.0
Two Wavelengths (λ1, λ2)
Reconstruction algorithm provides Δµ a
for each volume element (voxel) of finite element mesh
for each wavelength.
left occlusion
HbO 2 [µM]
15.
-8.0
-30.
-55.
-78.
For each voxel we get two equations:
.
λ1
λ1
λ1
-90.
Δµa = ε Hb Δ[Hb] + ε HbO2 Δ[HbO2 ]
0.4
-10.
Lt.
-20.
-34.
-40.
THb[µM]
12.
Lt.
λ 2 Δ[Hb] + ε λ 2
Δµaλ 2 = ε Hb
HbO2 Δ[HbO2 ]
ε := extinction coefficient (from literature)
15
Two Wavelengths
Movie
Δ Hb, HbO 2, THb (source 1, detector 12)
Reconstruction algorithm provides Δµa
for each volume element (voxel) of finite element mesh
for each wavelength.
posterior
λ
From this we can calculate changes in concentrations of
oxy-hemoglobin, Δ[Hb], and dexoy-hemoglobin, Δ[HbO 2],
for each voxel.
source 1
detector 12
β
anterior
λ 2 Δµ λ1 − ε λ1 Δµ λ 2
ε HbO
a
HbO2
a
Δ[Hb] = λ1 2 λ 2
λ 2 λ1
ε Hb ε HbO2 − ε Hb ε HbO2
ε λ1 Δµ λ 2 − ε λHb2 Δµaλ1
Δ[HbO2 ] = λ1 Hbλ 2 a
1
ε Hb ε HbO2 − ε λHb2 ε λHbO
2
Forepaw Stimulation
Right Forepaw Stimulation
lt.
rt.
50
-27.0 µM
Δ[HbO2]*
*Oxyhemoglobin
16
Reconstruction
Blood Volume
A.Y. Bluestone, M. Stewart, B. Lei, I.S. Kass, J. Lasker, G.S. Abdoulaev,
A.H. Hielscher, "Three-dimensional optical tomographic brain imaging in
small animals, Part I: Hypercapnia," Journal of Biomedical Optics 9(5),
pp. 1046-1062 (2004).
Cut 3
Cut 7
Cut 10
lt.
rt.
-0.003
For more details see:
0
0.004
ΔΤHb [mM]
Overview
• Introduction
X-ray vs optical tomography
• Model-based iterative image reconstruction
A.Y. Bluestone, M. Stewart, J. Lasker, G.S. Abdoulaev, A.H. Hielscher,
"Three-dimensional optical tomographic brain imaging in small animals,
Part II: Unilateral Carotid Occlusion," Journal of Biomedical Optics 9(5),
pp. 1063-1073 (2004).
A.Y. Bluestone, Kenichi Sakamoto, A.H. Hielscher, M. Stewart, “ThreeDimensional Optical Tomographic Brain Imaging during Kainic-AcidInduced Seizures in Rats,” in Physiologu, Function, and Structure from
Medical Images, A. Amini, A. Manduca, eds., SPIE-The International
Society for Optical Engineering, Proc. 5746, pp. 58-66 (2005).
www.bme.columbia.edu/biophotonics
Tumors in Mice
• Tumor is injected into mouse left kidney.
• Tumor continues to grow unless treated.
Basic concepts and mathematical background
• Instrumentation
Static Measurements
Dynamic Measurements
• Treatment with VEGF antagonist seeks to
stop angiogenesis and reverse tumor growth.
• Applications
Brain Imaging
Tumor Imaging
Fluorescence Imaging
17
Tumors in Mice
More Information:
• Untreated tumors: highly vascularized
Frischer
-Chiweshe A,
Kadenhe
Frischer JS,
JS, Huang
Huang JZ, Serur A, KadenheKadenhe-Chiweshe
A, McCrudden
McCrudden KW,
KW,
O'Toole
O'Toole K,
K, Holash
Holash J,
J, Yancopoulos
Yancopoulos GD,
GD, Yamashiro
Yamashiro DJ,
DJ, Kandel
Kandel JJ
JJ "Effects
"Effects of
of
potent
potent VEGF
VEGF blockade
blockade on
on experimental
experimental Wilms
Wilms tumor
tumor and
and its
its
persisting
persisting vasculature"
vasculature"
INTERNATIONAL
INTERNATIONAL JOURNAL
JOURNAL OF
OF ONCOLOGY
ONCOLOGY 25
25 (3):
(3): pp.
pp. 549-553
549-553 (2004).
(2004).
• Treated tumors: much less vascularized
• Currently:
Many mice are sacrificed to get tumor data Fluorescent staining
with Lectin (10 x)
• Only 1 time point per mouse
• We propose to use MRI and OT to study tumor
size and vasculature in vivo
fMRI vs Optical Tomography
fMRI
Spatial Resolution 0.1mm- 1mm
Optical Tomography
2mm - 10mm
Sensitive to
Hb, HbO2, cytochrome,
etc, blood volume,
scattering properties
Speed
Hb
(paramag.)
0.1 - 1Hz
~50 Hz
> $500.000
~ $100.000
Portability
no
yes
Continuous
Monitoring
no
yes
Cost
Combine high spatial resolution of fMRI and high speed and
sensitivity of optical tomography!
Huang
Huang JZ,
JZ, Frischer
Frischer JS,
JS, Serur
Serur A,
A, Kadenhe
Kadenhe A,
A, Yokoi
Yokoi A,
A, McCrudden
McCrudden KW,
KW, New
New T,
T,
O'Toole
O'Toole K,
K, Zabski
Zabski S,
S, Rudge
Rudge JS,
JS, Holash
Holash J,
J, Yancopoulos
Yancopoulos GD,
GD, Yamashiro
Yamashiro DJ,
DJ,
Kandel
Kandel JJ
JJ "Regression
"Regression of
of established
established tumors
tumors and
and metastases
metastases by
by potent
potent vascular
vascular
endothelial
blockade
endothelial growth
growth factor
factor blockade”
blockade””
PROCEEDINGS
PROCEEDINGS OF
OF THE
THE NATIONAL
NATIONAL ACADEMY
ACADEMY OF
OF SCIENCES
SCIENCES OF
OF THE
THE
UNITED
UNITED STATES
STATES OF
OF AMERICA
AMERICA 100
100 (13):
(13): 7785-7790
7785-7790 (2003)
(2003)
Glade-Bender
Glade-Bender J,
J, Kandel
Kandel JJ,
JJ, Yamashiro
Yamashiro DJ,
DJ, "VEGF
"VEGF blocking
blocking therapy
therapy in
in the
the
treatment
treatment of
of cancer”
cancer”
EXPERT
OPINION
ON
BIOLOGICAL
THERAPY
3
(2):
263-276
APR
EXPERT OPINION ON BIOLOGICAL THERAPY 3 (2): 263-276 APR 2003
2003
9.4 Tesla MRI (Bruker Avance 400)
Micro2.5 Imaging set
35mm diameter
Linearly polarized
Birdcage coil
Typical imaging time: 30 - 60 minutes (T1 sequence)
18
Optical Tomography Set Up
Step 1
Step 2
Lower mouse into
imaging head.
Add matching fluid
(Intralipid).
Step 3
Axial Slice
Optical
[HbT]
HbT]
(M)
MRI
Kidney
Back Muscle &
Spinal Cord
Illuminate with
light (Image!)
Tumor
Total Hemoglobin
Typical imaging time: 10 - 20 minutes
Combine high spatial resolution of fMRI and high speed and
sensitivity of optical tomography!
Coronal Slice
[HbT]
HbT]
Optical
Compare Untreated vs. Treated
MRI
(M)
Kidney
Untreated [HbT]
Treated [HbT]
Untreated tumor
has higher [HbT]
than treated tumor
because of higher
vascularization.
Tumor
Untreated tumor
has higher [Hb]
than treated tumor
because it is HbO 2
starved.
Total Hemoglobin
Untreated [Hb] (M)
Treated [Hb] (M)
19
For more details see:
Overview
J. Masciotti, G. Abdoulaev, J. Hur, J. Papa, J. Bae, J. Huang, D. Yamashiro,
J. Kandel, A.H. Hielscher, “Combined optical tomographic and magnetic
resonance imaging of tumor bearing mice,” in Optical Tomography and
Spectroscopy of Tissue VII, B. Chance, R.R. Alfano, B.J. Tromberg, M.
Tamura, E.M. Sevick-Muraca, eds., SPIE-The International Society for
Optical Engineering, Proc. 5693, pp. 74-81 (2005).
• Introduction
X-ray vs optical tomography
• Model-based iterative image reconstruction
Basic concepts and mathematical background
• Instrumentation
General optical imaging modalities
Dynamic optical tomography system
• Applications
www.bme.columbia.edu/biophotonics
Brain Imaging
Tumor Imaging
Molecular Fluorescence Imaging
Rheumatoid Arthritis
Molecular Imaging
Light
NIRF
molecular
probes
targets
mouse
without RA
transgenic mouse
with RA
Antigen: glucose-6-phosphate isomerase (GPI)
Mahmood,
Weissleder et al
MGH-CMIR
KRN transgene on the
Non transgenic B6xNOD.
(GPI) glycolytic enzynme is Antigen
B6xNOD F1 backgrou
the T cells and immunoglobins attack.
(K/BxN)
Only when GPI is expressed in synovial tissue rheumatoid arthritis develops
Developed fluorescent markers that shine when GPI is present/
20
Cancer Detection
Fluorescence Tomography
reconstruction of
absorption and scattering
profile µ(x,y)
µ(x,y)
reconstruction of
fluorescence source
profile S(x,y)
S(x,y)
light
source
light
source
Mfl
Fluorescence Tomography
1) Excitation λ x
Inverse Source Problem
Ω ⋅ ∇Ψ (r,Ω ) + ( µ a + µ s )Ψ(r,Ω ) = S (r,Ω ) + µ s ∫ p(Ω,Ω')Ψ(r,Ω')dΩ'
2) Emission λm
4π
φx
φm
[ W cm-2 ]
[ W cm-2 ]
1) Excitation λ x
(
)
Ω ⋅ ∇Ψ x + µ a x→ + µ a x→ m + µ s x Ψ x = S x + µ s x ∫ p( Ω,Ω')Ψ x ( Ω')dΩ'
4π
φ x = ∫ Ψ x (Ω' )dΩ'
4π
fluorophore
2) Emission λm
µ a x→ m absorption of
fluorophore
€
€
η
quantum yield
of fluorophore
(
)
Ω ⋅ ∇Ψ m + µ a m + µ s m Ψ m =
1
ηµ x→ m φ xx + µ s m ∫ p( Ω,Ω')Ψ m (Ω')dΩ'
4π a
4π
21
Model-Based Image Reconstruction
Model-Based Image Reconstruction
1) Excitation λ x
1) Excitation λ x
Forward Model
Forward Model
Experiment M
Prediction P
Prediction P
Inverse Model
2) Emission λm
φx
Forward Model
Experiment M
Inverse Model
µ a x→ m
µ a x→ m
€
€
Mouse Tomography
€
Model-Based Image Reconstruction
1) Excitation λ x
2) Emission λm
€
Forward Model
Prediction P
Inverse Model
µ a x→ m
φx
Experiment M
Forward Model
Prediction P
Experiment M
Inverse Model
µ a x→ m
Image
22
Mouse Tomography
For more details see:
A.K. Klose, V. Ntziachristos, A.H. Hielscher, "The inverse source problem
based on the radiative transfer equation in molecular optical imaging,"
J. of Computational Physics 202, pp. 323-345 (2005).
1 mm
A.K. Klose, A.H. Hielscher, "Fluorescence tomography with the equation
of radiative transfer for molecular imaging," Optics Letters 28(12), pp.
1019-1021 (2003).
3 mm
7 mm
c [au]
5 mm
0
9 mm
Summary
• Introduction
X-Ray Tomography vs Optical Tomography
• Model-based iterative image reconstruction
Basic concepts and mathematical background
• Instrumentation
General optical imaging modalities
Dynamic optical tomography system
• Applications
Brain Imaging
Tumor Imaging
Fluorescence Imaging
A.K. Klose, A.H. Hielscher, " Optical fluorescence tomography with the
equation of radiative transfer for molecular imaging," in Optical
Tomography and Spectroscopy of Tissue V, B. Chance, R.R. Alfano,
B.J. Tromberg, M. Tamura, E.M. Sevick-Muraca, eds., SPIE-The
International Society for Optical Engineering, Proc. 4955, pp. 219-225
(2003).
www.bme.columbia.edu/biophotonics
Acknowledgements I
• Students:
J. Masciotti, X. Gu, J. Hur, F. Provenzano, J. Lasker,
A. Bluestone, B. Moa-Anderson
• Postdoctoral Fellows:
A. Klose, G. Abdoulaev, J. Papa
• Collaborators:
Columbia
J. Kandel (Pediatrics & Surgery, Columbia)
D. Yamashiro (Pediatrics & Surgery, Columbia)
G. Bal (Applied Mathematics)
SUNY - Downstate
Mark Steward (Physiology & Pharmacology)
R.L. Barbour (Pathology)
C. Schmitz (NIRx Medical Technologies, Inc.)
23
Acknowledgements II
More Information
• National Institute of Arthritis and Musculoskeletal and
Skin Diseases (NIAMS) (RO1 AR46255-01 PI: Hielscher)
• National Institute for Biomedical Imaging and
Bioengineering (NIBIB) (R01 EB001900-01 PI: Hielscher
and 5 R33 CA 91807-3 PI: Ntziachristos)
• National Heart, Lung, and Blood Institute (NHLBI)
(SBIR 2R44-HL-61057-02)
• Whitaker Foundation (#98-0244 PI: Hielscher)
• Schering Research Foundation (PI: Klose)
www.bme.columbia.edu/biophotonics
.
24