2012 USABO Semifinal Exam Answers
advertisement
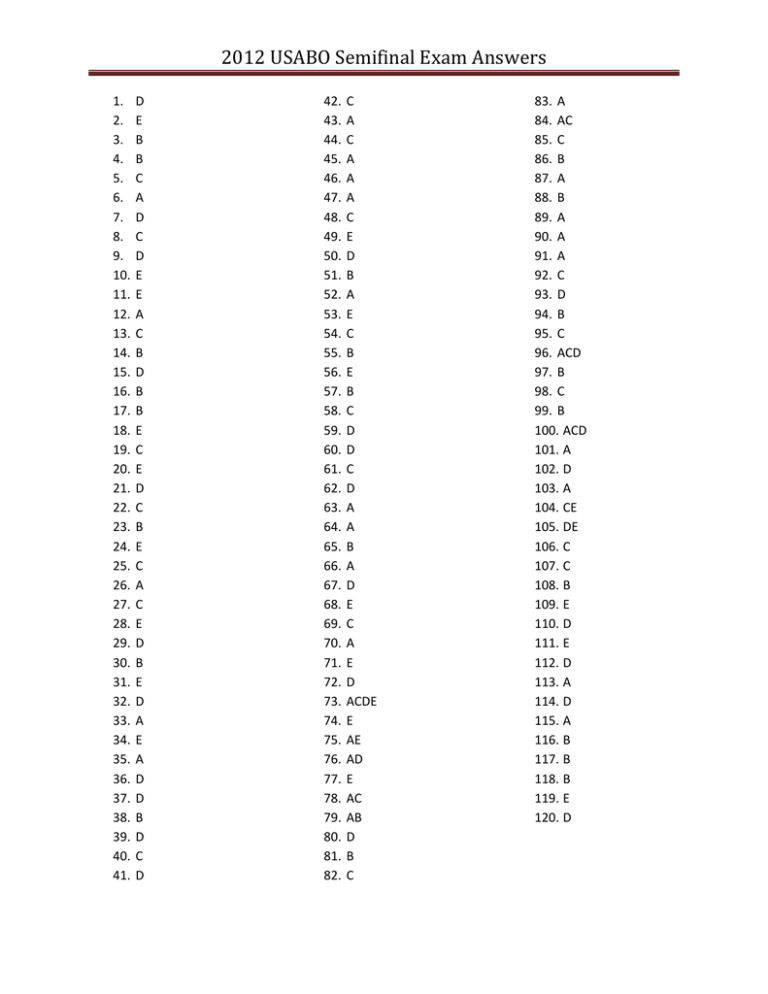
2012 USABO Semifinal Exam Answers 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. D E B B C A D C D E E A C B D B B E C E D C B E C A C E D B E D A E A D D B D C D 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. 70. 71. 72. 73. 74. 75. 76. 77. 78. 79. 80. 81. 82. C A C A A A C E D B A E C B E B C D D C D A A B A D E C A E D ACDE E AE AD E AC AB D B C 83. A 84. AC 85. C 86. B 87. A 88. B 89. A 90. A 91. A 92. C 93. D 94. B 95. C 96. ACD 97. B 98. C 99. B 100. ACD 101. A 102. D 103. A 104. CE 105. DE 106. C 107. C 108. B 109. E 110. D 111. E 112. D 113. A 114. D 115. A 116. B 117. B 118. B 119. E 120. D PART C Student Name____________________________ Student ID#___________________ Part C should be returned in its entirety with each student’s scantron. Place all answers to Part C, Questions 1 and 2, on these two pages. Additional sheets of paper may be used, if necessary. Be sure that each page has the Student’s Name and the Student’s ID#. Please staple all pages together. 2012 USABO Semifinal Part C 1. The diagram shows an apparatus made by a student to investigate the effect of temperature on the activity of ethanol fermentation of yeast. The conical flask contains 2.5 g yeast suspended in 2% sucrose solution. The meniscus moves down the glass tube (5 mL micropipette) during fermentation. The data shown below were collected at regular time intervals to assess the amount of suspension (mL) pushed in the glass tube due to CO2 accumulation Time (min) 1 2 3 4 5 4oC 0 0 0.1 0.2 0.3 10oC 0.2 1.0 1.9 3.1 4.0 20oC 0.4 1.3 2.2 3.3 NO RESULT 35oC 0.7 1.2 2.8 4.4 NO RESULT 55oC 0 0.1 0.2 0.3 0.4 1 A. Estimate the average rate of CO2 production (mL CO2/min) for the yeast suspension at 20oC using the values obtained in the period between 2 and 4 minutes. 1 ml/min B. Estimate the specific rate of CO2 generation [millimoles CO2/(min∙g)] at 20oC. 0.017 – 0.018 mmoles CO2/min∙g C. What would be the specific rate of ethanol accumulation [millimoles CH3CH2OH/(min∙g)], if the fermentation reaction follows the equation C6H12O6 2C2H5OH + 2CO2? 0.017 – 0.018 mmoles CH3OH /min∙g Partial points should be given if the student identifies that the answer to B and C will be the same, even if they don’t get the numbers right (or calculate any numbers at all). 2. Complete the following table about hormones. Hormone Endocrine Gland Chemical Class Progesterone Ovaries Steroid Thyroid-stimulating Anterior pituitary Glycoprotein hormone gland Luteinizing hormone Anterior pituitary Glycoprotein Follicle-stimulating hormone Calcitonin Prolactin Antidiuretic hormone Anterior pituitary gland Thyroid gland Anterior pituitary gland Posterior pituitary gland Glycoprotein Peptide Protein Peptide Regulated By FSH and LH Hypothalamic hormones Hypothalamic hormones Hypothalamic hormones Blood calcium level Hypothalamic hormones Water/salt balance We hope to see you as a Finalist!! 2