Problem Set 4 Solutions 3.20 MIT Dr. Anton Van Der Ven
advertisement
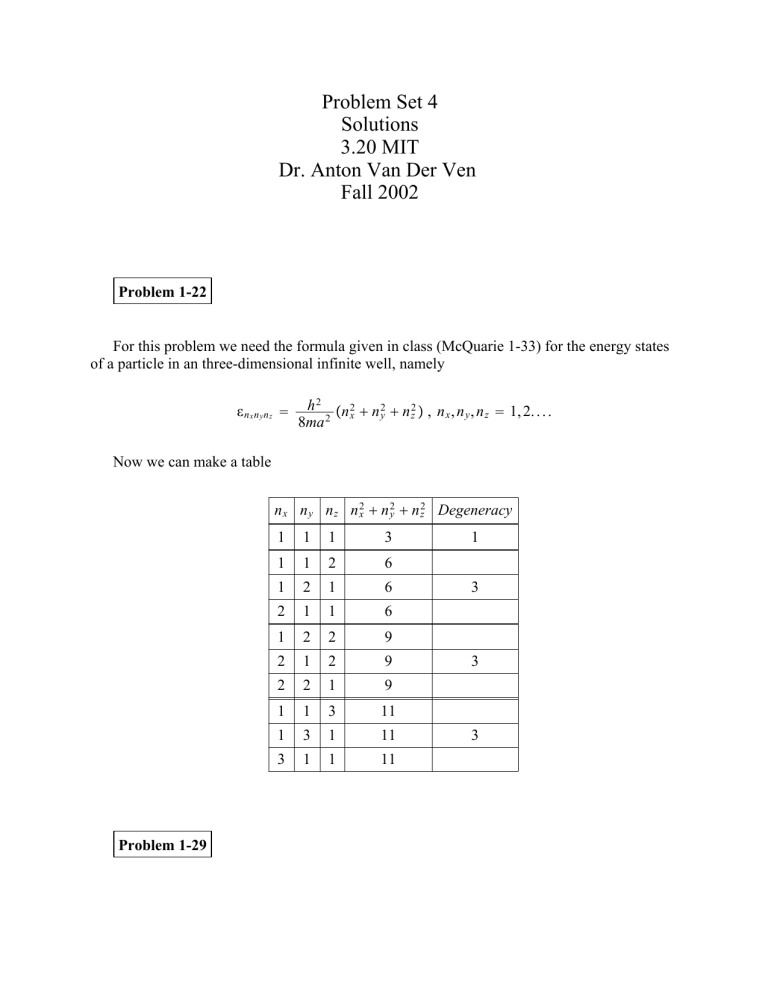
Problem Set 4 Solutions 3.20 MIT Dr. Anton Van Der Ven Fall 2002 Problem 1-22 For this problem we need the formula given in class (McQuarie 1-33) for the energy states of a particle in an three-dimensional infinite well, namely nxnynz h 2 n 2 n 2 n 2 , n x , n y , n z 1, 2. . . . x y z 8ma 2 Now we can make a table n x n y n z n 2x n 2y n 2z Degeneracy Problem 1-29 1 1 1 3 1 1 2 6 1 2 1 6 2 1 1 6 1 2 2 9 2 1 2 9 2 2 1 9 1 1 3 11 1 3 1 11 3 1 1 11 1 3 3 3 Start with the differential form for E dE TdS pdV E V We can use a Maxwell relation on T S V T S V T S V T p from FT, V T p T V So, E V T T p T V p Problem 1-41 Show that x x 2 x 2 x 2 x x 2 x 2 2x x x 2 x 2 2x x x 2 x 2 2 x x x 2 x 2 x 2 Problem 1-43 Here we have to plot the Gaussian px to see what happens as 0 1 2 exp x x 2 2 2 for several values of As 0, the function becomes sharper and sharper (remember the area under the curve is contrained to be 1, as we will see in problem 1-44a). Thus, the Gaussian approaches a delta function. Problem 1-44 Gaussian distribution is px (a) show pxdx 1 Let 2 1 exp x x 2 2 2 2 1 exp x x 2 2 2 dx u x x , then du 2 dx 2 So we now have 1 expu 2 du And using standard integral tables or the math software, it can be show that this integral is equal to 1. (b) n th central moment for n 0, 1, 2, and 3 For n 0 x x 0 1 see part a For n 1 x x x x pxdx x x x x 2 exp 2 2 2 dx Let u x x , then du 2 dx 2 Then x x 1 u expu 2 du 1 0 u expu 2 du 1 0 u expu 2 du I II For I let v u and dv du. Then 0 I 1 1 u expu 2 du 0 v expv 2 dv II Thus, 1 u expu 2 du 0 For n 2 x x 2 Let x x pxdx x x 2 x x 2 exp 2 2 2 dx u x x , then du 2 3 x x 1 2 2 u 2 expu 2 du dx 2 2 2 u 2 expu 2 du Now lets take a crack at this integral.... u 2 expu 2 du We have to do this by parts. Remembering how to do that.... yx y x yx y x yx yx For our case, let y 2u expu 2 du and x u 2 y expu 2 and x 1 2 So, u 2 expu 2 du u expu 2 2 1 2 0 expu 2 2 Which leaves us with, x x 2 2 For n 3 3 x x x x 3 pxdx x x 3 x x 2 exp 2 2 2 dx As before, let u x x , then du 2 dx 2 Then, 3 2 x x 2 u 3 expu 2 2 Since e u is a symmetric function around the origin and u 3 is an antisymmetric function around the origin, the integral of the product of the two functions is zero. 3 x x 0 1 0 2 (c) lim px lim 0 exp x x 2 2 2 The delta function is defined as x x , the delta function x a xdx a and xdx 1 So lets see if this works for our case lim 0 Let u xa 2 and du 1 exp x a 2 2 2 2 xdx dx 2 lim 1 0 expu 2 2 a Since 0 1 expu 2 a a expu 2 a Problem 1-49 Maximize WN 1 , N 2 , . . . . N m N! m Nj! j1 under the contraints that N j N and E j N j . Since the natural log is a monotonic function, we can maximize lnW. M ln WN 1 , N 2 , . . . . N m ln Using Sterling approximation, this can be written as N! m Nj! j1 N ln N N N j ln N j N j j j But the maximization must be constrained therefore we intoduce Lagrange multipliers M N ln N N N j ln N j N j j j Nj N EjNj N Remembering N j N and taking the derivative of M with respect to N j M N j ln N j 1 E j 0 ln N j 1 E j ln N j E j , with 1 N j exp expE j Problem 1-50 We want to show that the maximum of WN 1 , N 2 , . . . N m N! m Nj! j1 occurs for N 1 N 2 N s N s Maximize M ln W N ln N N N j ln N j N j subject to the constraint N j N. So we must use Lagrange multipliers: M N ln N N N j ln N j N j Nj N M ln N 1 0 j N j ln N j 1 N j exp1 But now we must determine s s j1 j1 N j exp1 S exp1 N exp1 So Nj N s N s Problem 1-51 Here we use Lagrange multipliers again with the constraint j P j 1. N M P j ln P j j1 N Pj 1 j1 Maximize M P j ln P j 1 0 P j exp1 Determine N P j N exp1 1 j1 exp1 1 N Thus Pj 1 N Problem 2-1 From stat mech we get E V And from thermo we have N, p p N,V E V N,T T p T N,V p To show why cont T lets see what happens if we let T where is a constant E V N, T p T p N,V or E V N,T but from a Maxwell relation we know E V T p T N,T p T N,V S V T S V N,T N,V p N,T and we can write p dE TdS PdV This statement violates the second law of thermodynamics because it implies that Q Q TdS T dS Problem 2-2 Given n! n!n n 1 ! n is n 1 n 1 ? n 1 is the value of n 1 that maximizes n. From problem 1-50, we know that n 1 n2 . n 1 is given by n n1 n1 n 1 0 n n 1 0 n! n 1 !nn 1 ! n! n 1 !nn 1 ! We also know that (given in the problem): n y 1 x n xn 1 n 1 0 n! n 1 !n n 1 ! If we take the derivative of y with respect to x we get n y n1 x n1 n1xn n 1 0 11 n! n 1 !n n 1 ! If we let x 1: n n2 n1 n1 n 1 0 Furthermore, n! n 1 !n n 1 ! 2-2-1 n 2n n! n 1 !n n 1 ! n 1 0 So if we put this all together back in (2-2-1) we get n n1 n1 n! n 1 !nn 1 ! n 1 0 n n1 n2 n n n 1 2 2 n! n 1 !nn 1 ! n 1 0 n 1 n 1 Problem 2-5 Show that S k P j ln P j We have the following three relations already S kT ln Q T N,V exp E j kT Pj k ln Q Q and Q exp j E j kT Starting with S we can write Q k ln Q kT S kT Q Q T S k Q S k Q ln j j Ej kT exp j exp E j kT Ej kT 2 exp E j kT E j kT k ln Q exp E j kT k ln Q k ln Q E j kT exp E j S k ln exp kT j Q k ln Q P j Since P j 1 S k P j ln exp j E j kT k ln Q j E j kT S k P j ln exp j k P j ln j 1 Q E j kT exp S k P j ln Pj Q j S k j P j ln P j Problem 2-8 Q E j expE j j E j expE j E j Q E Q Q ln Q ln Q Problem 2-10 First derive E kT 2 ln Q T N,V starting from the result of problem 2-8, namely E ln Q From the chain rule ln Q T E We know 1 kT so T kT 2 , thus ln Q T E kT 2 ln Q T We can check this by taking kT 2 kT 2 ln Q T kT N,V Q T kT 2 N,V 2 T N,V T N,V Q E exp kTj . kT Q N,V 2 T E exp kTj N,V Q E exp kTj Q Ej kT 2 The kT 2 cancels and we are left with kT 2 ln Q T N,V E E j exp kTj Q definition of E So we have shown again that indeed E kT 2 ln Q T . Now for the relation involving p p p j exp Q Ej kT E j V exp Ej kT Q p kT Q V Q kT ln Q V kT V E exp kTj j N,T Q N,T Problem 2-11 Starting with F kT ln Q (Remember F A S F T S kT ln Q T V,N p F V p kT ln Q V V,N k ln Q Eqn. 2-33 T,N Eqn. 2-32 T,N F E TS E F TS E kT ln Q T kT E kT 2 ln Q T ln Q T V,N k ln Q Eqn. 2-31 V,N Problem 2-13 E For a particle confined to a cube of length a we are asked to show p j 23 Vj . We can start with the equation for the energy states of a particle in an three-dimensional infinite well, namely Ej h 2 n 2 n 2 n 2 , n x , n y , n z 1, 2. . . . x y z 8ma 2 1 Remembering that a 3 V or a V 3 we can write E j in terms of V 2 3 2 E j h V n 2x n 2y n 2z 8m E j pj V 5 2 3 3 2 2 2 h V n 2x n 2y n 2z 2 1 h V n 2x n 2y n 2z 8m 3 8m 3 V Ej So we have 2 Ej 3 V pj and taking the ensemble average gives us p 2 E 3 V Problem 2-14 3N 2 2mkT h2 QN, V, T 1 N! VN For p , ln Q V p kT ln Q ln T.N 3N ln 2mkT 2 h2 p kTN V 1 N! N ln V Now for E E kT 2 ln Q T N,V kT 2 3N 2 2mk h2 2mkT h2 E 3N kT 2 Ideal gas equation of state is obtained when Q fTV N ln Q ln fT N ln V p kT ln Q V T.N kT ln fT V p NkT V kT T,N N ln V V Problem 2-15 We are given Q as Q substituting in 3N h 2kT exp h kT exp 1 exp U o kT h k Q 3N exp 2T 1 exp T exp U o kT To find c v we need to use the following two relations E kT 2 ln Q T cv N,V E T Using your favorite math package (Maple), we can get E as E 1 2 Uo e kT 3Nk e Uo kT 12Nk e U o k kT 9Nk e 2 kU o kT 1 e T 2U o e 2 and c v as cv E T 3 Now we can take the lim c v and we find that T kN 2 e T T 2 1 e T 2 Uo kT 4U o e U o k kT 2U o e 2 kU o kT k 2 e T N lim 3 T T 2 1 e T 2 3Nk