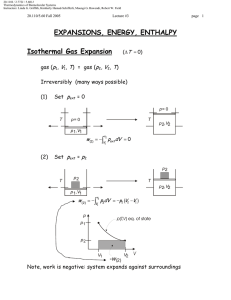
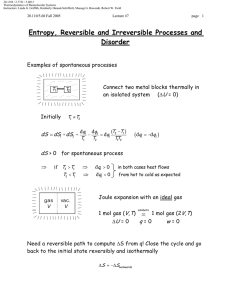
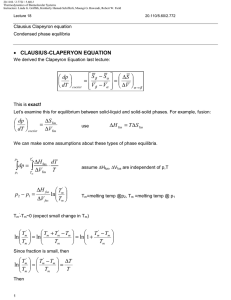
Document 13540041
advertisement

20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field Lecture 16 5.60/20.110/2.772 Absolute Entropy Third Law of thermodynamics • Absolute Entropies Absolute entropy of an ideal gas Start with fundamental equation dU = TdS − pdV dS = dU + pdV T for ideal gas: dU = CV dT and p = dS = nRT V CV dT nR + dV T V At constant T, dT=0 dST = pdV T dST = nRdV V For an ideal gas, pV = nRT At constant T d ( pV ) = d (nRT ) = 0 pdV = −Vdp plugging into dST: dST = − nRdp p This allows us to know how S(p) if T held constant. Integrate! 1 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field Lecture 16 5.60/20.110/2.772 For an arbitrary pressure p, p S(p,T ) = S( po ,T ) − ³ p o § p· nRdp = S(p o ,T ) − nRln ¨ o ¸ p ©p ¹ where po is some reference pressure which we set at 1 bar. S(p,T) = So(T) – nR lnp (p in bar) 0 S ( p, T ) = S (T ) − R ln p S ↓ as P↑ p S° But to finish, we still need S o (T ) ! o Suppose we had S ( 0 K ) (standard molar entropy at 0 Kelvin) dH = TdS + Vdp dH = C p dT for ideal gas C p dT = TdS + Vdp dS = 2 Cp T dT − V dp T 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field Lecture 16 5.60/20.110/2.772 Cp § ∂S · = Then using © ∂T ¹ p T o S (T ) . Integrating over dS eqn, assuming Cp constant over T range: we should be able to get T2 dS = Cp ³T T1 p2 dT − nR ³p p dp 1 So then §T · §p · ∆S = C p ln¨¨ 2 ¸¸ − nR ln¨¨ 2 ¸¸ © T1 ¹ © p1 ¹ for p = 1bar §T · = C p ln¨¨ 2 ¸¸ − nR ln p © T1 ¹ Given Cp, T1, p1, → T2, p2, can calculate ∆S. We will use T=0K as a reference point. Consider the following sequence of processes for the substance A: A(s,0K,1bar) = A(s,Tm,1bar) = A(l,Tm,1bar) = A(l,Tb,1bar) = A(g,Tb,1bar) = A(g,T,1bar) S (T,1bar) = S o (0K ) + ³ Tm 0 So(T) T b C p ()dT T C p (g)dT C p (s)dT ∆H fus ∆H vap + +³ + +³ Tm Tb T T T Tm Tb ∆S = ³ CpdT T Liquid boils, ∆S = ∆H fus Solid melts, ∆S = T 0 3 T ∆H vap T 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field Lecture 16 5.60/20.110/2.772 Since ∆S0 is positive for each of these processes, the entropy must have its smallest possible value o at 0 K. If we take S ( 0 K ) = zero for every pure substance in its crystalline solid state, then we could calculate the entropy at any other temperature. This leads us to the Third Law of Thermodynamics: • THIRD LAW: First expressed as Nernst's Heat Theorem: Nernst (1905): As T → 0 K , ∆S → 0 for all isothermal processes in condensed phases More general and useful formulation by M. Planck: Planck (1911): As T → 0 K , S → 0 for every chemically homogeneous substance in a perfect crystalline state Justification: ? @ It works! Statistical mechanics (5.62) allows us to calculate the o entropy and indeed predicts S ( 0 K ) = 0. This leads to the following interesting corollary: It is impossible to decrease the temperature of any system to T = 0 K in a finite number of steps. How can we rationalize this statement? Recall the fundamental equation, dU = T dS – p dV dU = Cv dT so For 1 mole of ideal gas, P = RT/V Cv dT = T dS – (RT/V) dV dS = Cv d (ln T) + R d (ln V) 4 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field Lecture 16 T2, 5.60/20.110/2.772 For a spontaneous adiabatic process which takes the system from T1 to a lower temperature ∆S = Cv ln (T2/T1) + R ln (V2/V1) ≥ 0 but if T2 = 0, Cv ln (T2/T1) equals minus infinity ! Therefore R ln (V2/V1) must be greater than plus infinity, which is impossible. Therefore no actual process can get you to T2 = 0 K. But you can get very very close! In W. Ketterle's experiments on "Bose Einstein Condensates" (recent MIT Nobel Prize in Physics), atoms are cooled to nanoKelvin temperatures (T = 10-9 K) … but not to 0 K ! _______________ Some apparent violations of the third law (but which are not !) Any disorder at T = 0 K gives rise to S > 0 • mixed crystals If have an unmixed crystal, N atoms in N sites: Ω= N! =1 N! S = k ln 1 = 0 But if mixed crystal: NA of A NB of B NA + NB = N Ω= N! N A! N B ! S = k ln Use Stirling’s approx: 5 N! N A! N B ! 20.110J / 2.772J / 5.601J Thermodynamics of Biomolecular Systems Instructors: Linda G. Griffith, Kimberly Hamad-Schifferli, Moungi G. Bawendi, Robert W. Field Lecture 16 5.60/20.110/2.772 ln N != N ln N − N S = k ( N ln N − N − N A ln N A + N A − N B ln N B + N B ) = − k (− N ln N + N A ln N A + N A + N B ln N B ) Using mole fractions: NA =xAN, NB =xBN [ ∆Smix = −nR X A ln X A + X B ln X B ] > 0 Always !!! Even at T=0K But a mixed crystal is not a pure substance, so the third law is not violated. • Any impurity or defect in a crystal also causes S > 0 at 0 K • Any orientational or conformational degeneracies such as in a molecular crystal causes S > 0 at 0 K, for example in a carbon monoxide crystal, two orientations are possible: CO CO CO CO CO CO CO CO CO CO CO CO OC CO CO CO CO OC CO CO CO CO CO CO CO CO CO CO 6