Chemistry 351L Wet Lab
advertisement
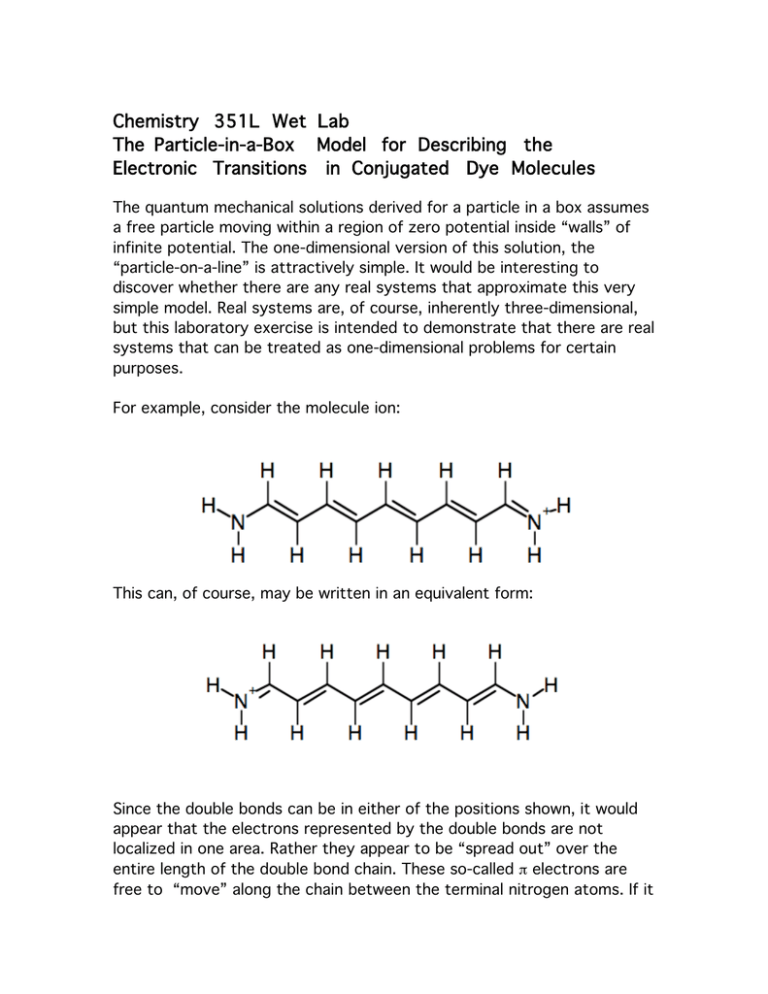
Chemistry 351L Wet Lab The Particle-in-a-Box Model for Describing the Electronic Transitions in Conjugated Dye Molecules The quantum mechanical solutions derived for a particle in a box assumes a free particle moving within a region of zero potential inside “walls” of infinite potential. The one-dimensional version of this solution, the “particle-on-a-line” is attractively simple. It would be interesting to discover whether there are any real systems that approximate this very simple model. Real systems are, of course, inherently three-dimensional, but this laboratory exercise is intended to demonstrate that there are real systems that can be treated as one-dimensional problems for certain purposes. For example, consider the molecule ion: This can, of course, may be written in an equivalent form: Since the double bonds can be in either of the positions shown, it would appear that the electrons represented by the double bonds are not localized in one area. Rather they appear to be “spread out” over the entire length of the double bond chain. These so-called π electrons are free to “move” along the chain between the terminal nitrogen atoms. If it is assumed that the potential energy influencing the electrons along the chain due to the carbon centers is constant over the length of the chain, this potential may, for convenience, be set at zero. Then this problem is similar to the one-dimensional particle that is free to move along the x direction between the limits x=0 and x=L. In the real molecule analog, the electrons are free to move along the chain (x) between the limits prescribed by the length of the chain. As we learned in class, the allowed energy values and the eigenfunctions are thus: Unlike the class example where a single particle was confined to the 1D box, in the molecular analogue there are a number of electrons confined to one box. (We are assuming that the electrons in this system do not interact with one another excepting, as seen below, through their spin). Each of these particles must be described by an eigenfunction. Because of spin, which we have not yet introduced, but with which you are familiar from previous courses, there is a maximum of two electrons that can have the same spatial eigenfunction. In the molecular ion shown above, there are a total of 12 π-electrons, one each from the 9 carbon atoms and three from the two nitrogen atoms. Hence these 12 electrons will occupy first 6 wavefunctions given by ψn (n = 1, 2,….6) each holding 2 electrons (one spin up and one spin down). In the more general case of N electrons in a “conjugated” π systems (meaning alternating single and double C-C bonds), the first N/2 (assuming N is even) energy levels would be occupied. PRE-LAB Question # 1. Use Mathematica to graph the potential corresponding to a box of length 10 where the potential outside the box is infinite and inside the box is zero. Now superimpose on this potential the first six energy levels for a particle moving in this box. Make your unit of energy equal to h2/8mL2. Superimpose on each energy level the corresponding probability distribution. Now in someway depict that the first six energy states are occupied and the sixth is not (consider using dashed lines or something of that sort. Finally, provide an appropriate caption for your plot to explain its meaning to someone else. Your plot should look a bit like Figure 3.2 in your text. In absorption spectroscopy, an electron in a given energy level absorbs a photon of a specific energy and is consequently elevated to a higher energy level. PRE-LAB Question # 2. Explain why one will only see a transition if the energy of the photon is equivalent to the energy difference between states For example, in our case above, one of the electrons in the 6th energy level could be excited to, say, the 7th level if the enrgy of the incoming photon were equal to 13 h2/8mL2. PRE-LAB Question # 3. Explain how the preceding energy was derived. We can write a general expression for a transition between an energy level ni (the initial n-value of an electron) to nf (the empty level). The energy change would be: ΔE = [h2/8mL2](nf2 – n i2 ) If we consider transitions from the highest occupied level to the lowest unoccupied level (as in our example above) and we let N be the number of π-electrons in the molecule, then ni = N/2 and n f = (N/2 + 1), hence, ΔE = hν = hc/λ = [h2/8mL2](N+1) where ΔE is the energy of the photon necessary to cause the transition. PRE-LAB Question # 4. Write a Mathematica function that gives the wavelength of a photon (in nm) necessary to cause a transition of an electron in the nth energy state to the mth when in a box of length of L. That is, there will be three inputs to your function—n, m, and L. For molecules such as the one above, N is equal to the number of carbon atoms in the chain (since each carbon atom contributes one electron) plus 3 (each N atom would contribute 2 electrons, but one electron has been lost to form the ion), so, N = nC + 3. The parameter L is just the length of the “box.” It can be expressed as the number of C-C bonds (which is the number of carbon atoms minus one) times the average length of the C-C bonds, plus the number of C-N bonds times the length of the C-N bond, plus some unknown distance representing the penetration of the wave function beyond each end of the conjugated chain. So, L = (nC-1)lCC + 2lCN + 2p where lCC is the length of the C-C bond, lCN is the length of the C-N bond and p is the (unknown) penetration at each end. PRE-LAB Question # 5. Write a Mathematica function to calculate the wavelength, λ, of an absorbed photon in terms of nC, lCC, lCN and p. Note that these parameters give you nothing more than L, n, and m you used in Question 4. Thus you might want to thnk about combining functions to achieve this end. In the equation you derived in pre-lab question #5, there are four unknown quantities that must be determined in order to estimate the wavelength of absorbed photons for a particular molecule. The first, the number of carbon atoms, nC, can be determined from the molecular structure. The other parameters, lCC, lCN, and p are difficult to determine exactly for these molecules, but appropriate values can be estimated from experiment, as will be shown below. The experiments involve the comparison of experimental and predicted absorption wavelengths for several dyes. The results will then be used to predict the absorption wavelength for a new molecule. The structures of the three dyes to be used in these experiments are shown below: As a first approximation, we will assume that the value of lCC in these molecules is the same as in benzene, a somewhat similar delocalized system, where lCC = 0.140 nm. The value of lCN will be assumed to be similar to that in pyridine, where lCN = 0.134 nm. Since the value of p is difficult to assess, we will assume a range of p values ranging from p= 0 to p = 0.140 nm. Using this range of values and your Mathematica functions, plot the wavelength of the photon needed to cause the transition versus p. Having done this, you will follow the procedures outlined below to obtain the absorption spectrum of dye # 1. From the wavelength of the absorption maximum (λmax) observed for this dye you will use your graph to pinpoint the value of p that gives the experimentally observed value of the absorption maximum. Now using this value of p, you can estimate the wavelength of the absorption maximum for dye # 2 and for dye # 3 and compare this to your measured value. Comment on any differences observed and propose additional changes to the model that might give a better estimate of (λmax). Also comment on the origin of this penetration depth in terms of the particle-on-a-line quantum mechanical model. Is reasonable to assume that the value of this parameter should be the same for all of the molecules tested? Why or why not? When considering this question, take a look at the problem given in the practice test of a particle in a finite square well.







