Association of the CYP2C19 *2 ... restenosis in patients undergoing coronary stenting
advertisement
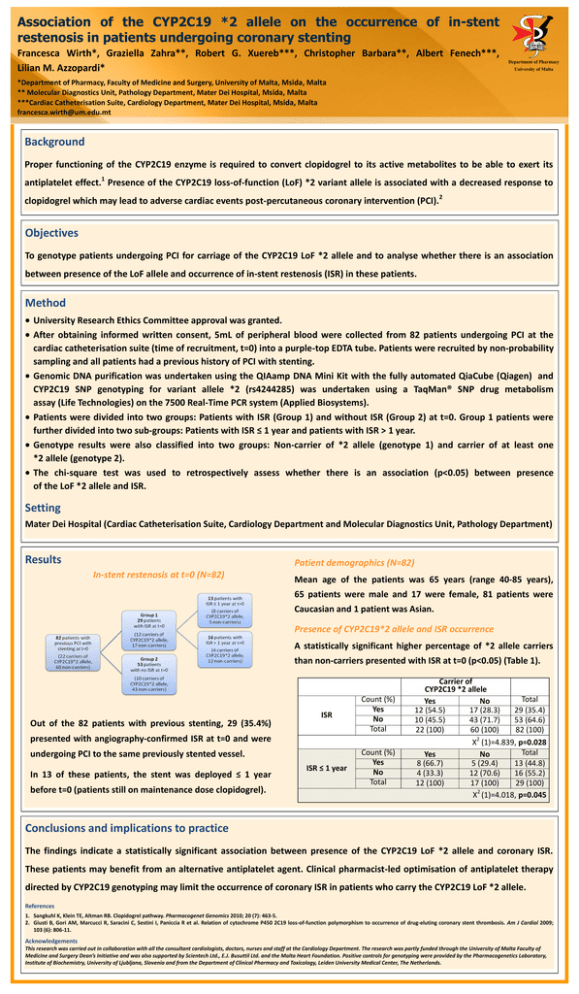
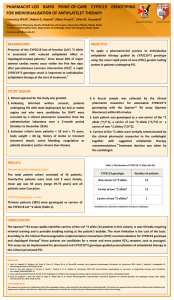
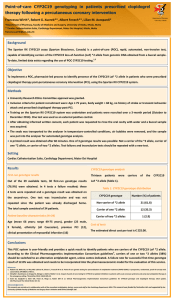
DEPARTMENT OF PHARM ACY UNIVERSI TY OF MA LTA Association of the CYP2C19 *2 allele on the occurrence of in-stent restenosis in patients undergoing coronary stenting Francesca Wirth*, Graziella Zahra**, Robert G. Xuereb***, Christopher Barbara**, Albert Fenech***, Lilian M. Azzopardi* Department of Pharmacy University of Malta *Department of Pharmacy, Faculty of Medicine and Surgery, University of Malta, Msida, Malta ** Molecular Diagnostics Unit, Pathology Department, Mater Dei Hospital, Msida, Malta ***Cardiac Catheterisation Suite, Cardiology Department, Mater Dei Hospital, Msida, Malta francesca.wirth@um.edu.mt Background Proper functioning of the CYP2C19 enzyme is required to convert clopidogrel to its active metabolites to be able to exert its 1 antiplatelet effect. Presence of the CYP2C19 loss-of-function (LoF) *2 variant allele is associated with a decreased response to clopidogrel which may lead to adverse cardiac events post-percutaneous coronary intervention (PCI). 2 Objectives To genotype patients undergoing PCI for carriage of the CYP2C19 LoF *2 allele and to analyse whether there is an association between presence of the LoF allele and occurrence of in-stent restenosis (ISR) in these patients. Method University Research Ethics Committee approval was granted. After obtaining informed written consent, 5mL of peripheral blood were collected from 82 patients undergoing PCI at the cardiac catheterisation suite (time of recruitment, t=0) into a purple-top EDTA tube. Patients were recruited by non-probability sampling and all patients had a previous history of PCI with stenting. Genomic DNA purification was undertaken using the QIAamp DNA Mini Kit with the fully automated QiaCube (Qiagen) and CYP2C19 SNP genotyping for variant allele *2 (rs4244285) was undertaken using a TaqMan® SNP drug metabolism assay (Life Technologies) on the 7500 Real-Time PCR system (Applied Biosystems). Patients were divided into two groups: Patients with ISR (Group 1) and without ISR (Group 2) at t=0. Group 1 patients were further divided into two sub-groups: Patients with ISR ≤ 1 year and patients with ISR > 1 year. Genotype results were also classified into two groups: Non-carrier of *2 allele (genotype 1) and carrier of at least one *2 allele (genotype 2). The chi-square test was used to retrospectively assess whether there is an association (p<0.05) between presence of the LoF *2 allele and ISR. Setting Mater Dei Hospital (Cardiac Catheterisation Suite, Cardiology Department and Molecular Diagnostics Unit, Pathology Department) Results Patient demographics (N=82) In-stent restenosis at t=0 (N=82) Mean age of the patients was 65 years (range 40-85 years), 65 patients were male and 17 were female, 81 patients were Caucasian and 1 patient was Asian. Presence of CYP2C19*2 allele and ISR occurrence A statistically significant higher percentage of *2 allele carriers than non-carriers presented with ISR at t=0 (p<0.05) (Table 1). Out of the 82 patients with previous stenting, 29 (35.4%) ISR Count (%) Yes No Total ISR ≤ 1 year Count (%) Yes No Total presented with angiography-confirmed ISR at t=0 and were undergoing PCI to the same previously stented vessel. In 13 of these patients, the stent was deployed ≤ 1 year before t=0 (patients still on maintenance dose clopidogrel). Carrier of CYP2C19 *2 allele Total Yes No 12 (54.5) 17 (28.3) 29 (35.4) 10 (45.5) 43 (71.7) 53 (64.6) 22 (100) 60 (100) 82 (100) 2 X (1)=4.839, p=0.028 Total Yes No 8 (66.7) 5 (29.4) 13 (44.8) 4 (33.3) 12 (70.6) 16 (55.2) 12 (100) 17 (100) 29 (100) 2 X (1)=4.018, p=0.045 Conclusions and implications to practice The findings indicate a statistically significant association between presence of the CYP2C19 LoF *2 allele and coronary ISR. These patients may benefit from an alternative antiplatelet agent. Clinical pharmacist-led optimisation of antiplatelet therapy directed by CYP2C19 genotyping may limit the occurrence of coronary ISR in patients who carry the CYP2C19 LoF *2 allele. References 1. Sangkuhl K, Klein TE, Altman RB. Clopidogrel pathway. Pharmacogenet Genomics 2010; 20 (7): 463-5. 2. Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R et al. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am J Cardiol 2009; 103 (6): 806-11. Acknowledgements This research was carried out in collaboration with all the consultant cardiologists, doctors, nurses and staff at the Cardiology Department. The research was partly funded through the University of Malta Faculty of Medicine and Surgery Dean’s Initiative and was also supported by Scientech Ltd., E.J. Busuttil Ltd. and the Malta Heart Foundation. Positive controls for genotyping were provided by the Pharmacogenetics Laboratory, Institute of Biochemistry, University of Ljubljana, Slovenia and from the Department of Clinical Pharmacy and Toxicology, Leiden University Medical Center, The Netherlands.